Successful treatment of gynecologic malignancy with trastuzumab deruxtecan in three patients with HER2 mutations
- PMID: 41158157
- PMCID: PMC12554900
- DOI: 10.1016/j.gore.2025.101974
Successful treatment of gynecologic malignancy with trastuzumab deruxtecan in three patients with HER2 mutations
Abstract
Objectives: Trastuzumab deruxtecan is an antibody-drug conjugate that was recently FDA-approved as second-line therapy for advanced solid tumors expressing HER2 by immunohistochemistry at the 3+ level. Clinical trials have demonstrated trastuzumab deruxtecan's promise as a treatment for advanced gynecologic malignancies, which often have poor prognoses and limited treatment options. Here, we present three HER2 negative (IHC 0) or untested patients with HER2-mutated gynecologic cancers who derived significant benefit from trastuzumab deruxtecan.
Methods: Consent from patients to carry out this research was obtained, and their cases were reviewed via the electronic medical record. Relevant clinical, surgical, pathologic, and imaging data are described in this report.
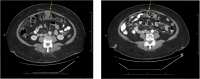
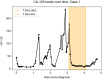
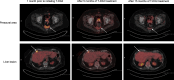
Results: These patients with HER2-mutated disease benefited from trastuzumab deruxtecan despite being HER2 negative (IHC 0) or untested by immunohistochemistry and therefore not meeting current criteria for FDA-approved treatment with this drug. The first case describes a 65-year-old woman with recurrent high grade endometrial carcinoma who had a progression-free survival of over 12 months with trastuzumab deruxtecan. In the second case, a 76-year-old woman with recurrent low grade serous ovarian carcinoma received trastuzumab deruxtecan for over two years and continues the treatment at the time of this article's submission. The third case describes a 52-year-old woman with recurrent cervical mesonephric adenocarcinoma who had a 15-month long partial response to trastuzumab deruxtecan.
Conclusions: These cases illustrate the potential for trastuzumab deruxtecan to be useful among a broader range of patients than current guidelines include and suggest that comprehensive genomic testing is necessary to help identify patients whose disease may be susceptible to HER2-targeted therapies.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
