Establishment of chicken muscle and adipogenic cell cultures for cultivated meat production
- PMID: 41158655
- PMCID: PMC12554555
- DOI: 10.3389/fnut.2025.1648935
Establishment of chicken muscle and adipogenic cell cultures for cultivated meat production
Abstract
Introduction: Cultured meat seeks to replicate the sensory and nutritional attributes l of conventional meat by developing structured muscle tissue using cell culture. This study focuses on the culture of chicken embryonic and muscle-derived mesenchymal stem cells (MSCs) to derive muscle, and fat, optimizing conditions for differentiation and integration.
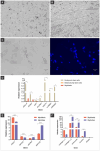
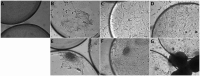
Methods: We utilized monolayer and three-dimensional microcarrier-based cultures to produce muscle fibers and adipocytes while maintaining the extracellular matrix (ECM) integrity essential for tissue cohesion. Key pluripotency and myogenic markers (e.g., cOCT4, cMYOD, cMYH1E) were analyzed during differentiation, revealing dynamic gene expression patterns that underscore myogenesis.
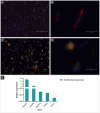
Results: Myoblast differentiation into mature myotubes demonstrated decreased cPAX7 (-35%) and increased cMYMK (+67%), confirming lineage commitment and muscle fiber formation. Adipogenesis was induced in embryonic MSCs using food-grade lecithin, which activated PPARγ, C/EBPα, and FABP4,resulting in robust lipid droplet accumulation. To scale production, microcarriers facilitated cell proliferation, while transglutaminase-based stabilization enabled the formation of three-dimensional tissue structures comparable to native meat.
Conclusion: Our findings highlight advances in culture protocols, genotypic and phenotypic expression analyses of multinucleated chicken muscle and adipocyte cells for cultured meat production.
Keywords: adipogenesis; biomass; chicken cells; cultured meat; myogenesis.
Copyright © 2025 Haach, Silveira, Peixoto, Sá, Gressler, Feddern, Ibelli, Silva and Bastos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Food, Nations (FAO) AO of the U (2023). Organization (WHO) WH Food safety aspects of cell-based food. Food safety aspects of cell-based food FAO; WHO.
-
- Feddern V, Bastos APA, Gressler V, Marques DMC, Ferreira FC, Rodrigues CAV, et al. Cell lines for cultivated meat production. Cultiv Meat. (2024):29–54. doi: 10.1007/978-3-031-55968-6_3 - DOI
-
- Bhat ZF, Bhat H, Pathak V. Prospects for in vitro cultured meat - a future harvest. Princ Tissue Eng Fourth Ed. (2013) 2013:1663–83. doi: 10.1016/B978-0-12-398358-9.00079-3 - DOI
-
- Jairath G, Mal G, Gopinath D, Singh B. An holistic approach to access the viability of cultured meat: a review. Trends Food Sci Technol. (2021) 110:700–10. doi: 10.1016/j.tifs.2021.02.024 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

