The biomechanical signature of tumor invasion
- PMID: 41158752
- PMCID: PMC12557592
- DOI: 10.1016/j.gendis.2025.101771
The biomechanical signature of tumor invasion
Abstract
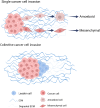
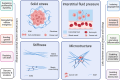
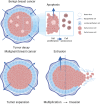
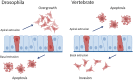
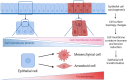
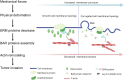
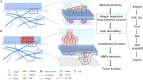
Tumor cell invasion is the key driver of metastatic dissemination, resulting in the development and progression of metastatic tumors at secondary sites, and remains the major cause of cancer-related death. Recent studies suggest that, in addition to protease-mediated degradation and chemotaxis-stimulated migration, tumor invasion is significantly influenced by physical surroundings. How tumor cells decode information about their shape deformation under mechanical stress and adapt their dynamic behavior to escape the confined regions remains largely unknown. This review highlights recent findings that illustrate mechanical cues in confined tumor microenvironment contribute to tumor progression. We also systematically discuss the role of compression-induced deformation in cell membrane topology and cytoskeletal remodeling, as well as its biophysical mechanisms in regulating tumor invasion from a biomechanical perspective.
Keywords: Actin remodeling; Mechanical forces; Mechanical memory; Microenvironment; Tumor invasion.
© 2025 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltdé.
Conflict of interest statement
The authors declared no conflict of interests.
Figures



References
-
- Bray F., Laversanne M., Sung H., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. - PubMed
-
- Githaka J.M., Pirayeshfard L., Goping I.S. Cancer invasion and metastasis: insights from murine pubertal mammary gland morphogenesis. Biochim Biophys Acta Gen Subj. 2023;1867(8) - PubMed
-
- Brabletz T., Kalluri R., Nieto M.A., Weinberg R.A. EMT in cancer. Nat Rev Cancer. 2018;18(2):128–134. - PubMed
-
- de Visser K.E., Joyce J.A. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403. - PubMed
-
- Raudenská M., Petrláková K., Juriňáková T., et al. Engine shutdown: migrastatic strategies and prevention of metastases. Trends Cancer. 2023;9(4):293–308. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

