Systematic data capture reduces the need for source data verification: exploratory analysis from a phase 2 multicenter randomized controlled platform trial
- PMID: 41162684
- PMCID: PMC12572212
- DOI: 10.1038/s43856-025-01126-9
Systematic data capture reduces the need for source data verification: exploratory analysis from a phase 2 multicenter randomized controlled platform trial
Abstract
Background: The COVID-19 pandemic gave rise to clinical trials focused on systematic, accurate primary data capture, and reduced reliance on source data verification (SDV). Here, we report on a natural experiment that allowed us to assess the quality, cost, and impact of this approach compared to traditional SDV.
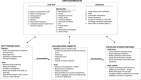
Methods: The I-SPY COVID trial (NCT04488081) was a multicenter, open-label, platform trial that employed a streamlined daily checklist, daily capture of labs and medications, and centralized monitoring to ensure accurate data collection in lieu of SDV. The trial enrolled 1,111 patients in 11 drug arms with severe COVID-19. After the trial arms were closed, extensive retrospective SDV was performed on 333 (30.0%) patients, including 10,101 of 44,486 (23%) electronic case report forms (eCRFs), allowing us to evaluate the impact of our strategy on data integrity, outcomes, and costs.
Results: We find that retrospective SDV results in changes to 0.36% (1,234 / 340,532) of data fields. It results in no changes to the type of outcome recorded (death, recovery, or censored), but changes in the day of recovery in 9 instances, by a median of 2 days (range 1-7). Two additional AEs are added during SDV that had not previously been captured. Costs associated with retrospective SDV of 23% eCRFs are 61,073 person-hours at a cost of $6.1 M.
Conclusions: Extensive SDV does not change any results or conclusions of the I-SPY COVID trial, which was designed with a systematic strategy for data capture, monitoring, and safety. This strategy could improve the efficiency of clinical trials and eliminate the need for manual SDV.
Plain language summary
This study aimed to determine whether using a systematic approach to record clinical trial data accurately in the first place would reduce the need to re-check the data for errors later. The approach combined automated transfer of laboratory data, simplified electronic forms for clinical trial staff to complete and ongoing monitoring for safety by a committee of physicians. Re-checking the data after the trial had completed, known as “source data verification” or SDV, revealed very few errors and none that changed the result or interpretation of the clinical trial’s findings. Because SDV is very time consuming and costly, this study may provide a way to help reduce the overall costs of clinical trials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: Ali B. Abbasi reports meeting travel reimbursements from Duke Clinical Research Institute and Johnson & Johnson. Kathleen D. Liu is an employee of the study sponsor, Quantum Leap Healthcare Collaborative, has received consulting fees from Baxter, Seastar Biomedical, AM Pharma, Biomerieux and UpToDate, acts in a paid advisory role to BOA Medical and Novartis, and holds securities in Amgen. Derek W. Russell received support from NIH Grant K08HL148514, grants from ABSS Solutions, Inc., the CHEST Foundation, and the NHLBI Prevention and Early Treatment of Lung Injury PETAL Network. He acts in an advisory capacity to Direct Biologics, Inc., and holds securities in Achieve Life Sciences. D. Clark Files has received consulting fees from Global Blood Therapeutics and Direct Biologics. Karl W. Thomas has received royalties from UpToDate, payments for expert testimony from Lewisbrisbois, Rushton, Stakey, Kohnston & Garrett PA and Phelan Tucker Law LLP, and holds securities in Bristol-Myers Squibb, Doximity, Gilead Sciences Inc, Johnson & Johnson and Pfizer. Fady Yousef has received payments for expert testimony from Independent Medical Examination and is a paid member of DSMB for Wuhan AltaScience. Martin Eklund has received consultancy fees from the study sponsor Quantum Leap Healthcare Collaborative, honoraria from Ipsen and Janssen, and holds securities in A3P Biomedical AB and Clinsight AB. Laura J. Esserman is a Medical Advisory Panel member for Blue Cross Blue Shield, sits on the board of directors of the study sponsor, Quantum Leap Healthcare Collaborative, and has received consulting fees from UpToDate. All other authors have nothing to disclose. Quantum Leap Healthcare Collaborative (QLHC) is a 501(c)3 not for profit organization and sponsor of the I-SPY COVID trial. QLHC provided a range of services to support the study, including all data and safety management, regulatory functions, and fundraising. For this manuscript, QLHC provided all relevant data, but played no part in drafting its content or conclusions.
Figures

References
-
- May, M. Clinical trial costs go under the microscope. Nat. Med. 10.1038/d41591-019-00008-7 (2019).
-
- Getz, K. A. & Campo, R. A. Trends in clinical trial design complexity. Nat. Rev. Drug Discov.16, 307–307 (2017). - PubMed
-
- Sertkaya, A., Birkenbach, A., Berlind, A. & Eyraud, J. Examination of Clinical Trial Costs and Barriers for Drug Development. https://aspe.hhs.gov/reports/examination-clinical-trial-costs-barriers-d... (2014).
-
- Institute of Medicine (US) Forum on Drug Discovery, Development and Translation. Transforming Clinical Research in the United States. (National Academies Press (US), Washington, DC, 2010). 10.17226/12900. - PubMed
LinkOut - more resources
Full Text Sources

