Ultrasound-driven programmable artificial muscles
- PMID: 41162719
- PMCID: PMC12571877
- DOI: 10.1038/s41586-025-09650-3
Ultrasound-driven programmable artificial muscles
Abstract
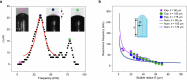
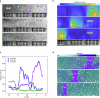
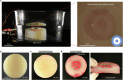
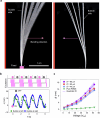
Muscular systems1, the fundamental components of mobility in animals, have sparked innovations across technological and medical fields2,3. Yet artificial muscles suffer from dynamic programmability, scalability and responsiveness owing to complex actuation mechanisms and demanding material requirements. Here we introduce a design paradigm for artificial muscles, utilizing more than 10,000 microbubbles with targeted ultrasound activation. These microbubbles are engineered with precise dimensions that correspond to distinct resonance frequencies. When stimulated by a sweeping-frequency ultrasound, microbubble arrays in the artificial muscle undergo selective oscillations and generate distributed point thrusts, enabling the muscle to achieve programmable deformation with remarkable attributes: a high compactness of approximately 3,000 microbubbles per mm2, a low weight of 0.047 mg mm-2, a substantial force intensity of approximately 7.6 μN mm-2 and fast response (sub-100 ms during gripping). Moreover, they offer good scalability (from micrometre to centimetre scale), exceptional compliance and many degrees of freedom. We support our approach with a theoretical model and demonstrate applications spanning flexible organism manipulation, conformable robotic skins for adding mobility to static objects and conformally attaching to ex vivo porcine organs, and biomimetic stingraybots for propulsion within ex vivo biological environments. The customizable artificial muscles could offer both immediate and long-term impact on soft robotics, wearable technologies, haptics and biomedical instrumentation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









References
-
- Gordon, M. S. et al. Animal Locomotion: Physical Principles and Adaptations (CRC Press, 2017).
-
- Zhang, J. et al. Robotic artificial muscles: current progress and future perspectives. IEEE Trans. Robot.35, 761–781 (2019).
-
- Laschi, C., Mazzolai, B. & Cianchetti, M. Soft robotics: technologies and systems pushing the boundaries of robot abilities. Sci. Robot.1, eaah3690 (2016). - PubMed
-
- Rus, D. & Tolley, M. T. Design, fabrication and control of soft robots. Nature521, 467–475 (2015). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

