Building wet planets through high-pressure magma-hydrogen reactions
- PMID: 41162720
- PMCID: PMC12571897
- DOI: 10.1038/s41586-025-09630-7
Building wet planets through high-pressure magma-hydrogen reactions
Abstract
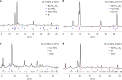
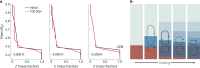
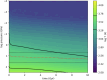
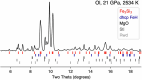
Close-in transiting sub-Neptunes are abundant in our Galaxy1. Planetary interior models based on their observed radius-mass relationship suggest that sub-Neptunes contain a discernible amount of either hydrogen (dry planets) or water (wet planets) blanketing a core composed of rocks and metal2. Water-rich sub-Neptunes have been believed to form farther from the star and then migrate inwards to their present orbits3. Here we report experimental evidence of reactions between warm, dense hydrogen fluid and silicate melt that release silicon from the magma to form alloys and hydrides at high pressures. We found that oxygen liberated from the silicate melt reacts with hydrogen, producing an appreciable amount of water up to a few tens of weight per cent, which is much greater than previously predicted based on low-pressure ideal gas extrapolation4,5. Consequently, these reactions can generate a spectrum of water contents in hydrogen-rich planets, with the potential to reach water-rich compositions for some sub-Neptunes, implying an evolutionary relationship between hydrogen-rich and water-rich planets. Therefore, detection of a large amount of water in exoplanet atmospheres may not be the optimal evidence for planet migration in the protoplanetary disk, calling into question the assumed link between composition and planet formation location.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




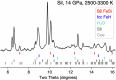

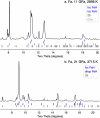
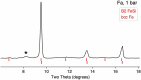
References
-
- Fulton, B. J. et al. The California-Kepler survey. III. A gap in the radius distribution of small planets. Astron. J.154, 109 (2017).
-
- Bitsch, B. et al. Dry or water world? How the water contents of inner sub-Neptunes constrain giant planet formation and the location of the water ice line. Astron. Astrophys.649, L5 (2021).
-
- Misener, W., Schlichting, H. E. & Young, E. D. Atmospheres as windows into sub-Neptune interiors: coupled chemistry and structure of hydrogen–silane–water envelopes. Mon. Not. R. Astron. Soc.524, 981–992 (2023).
-
- Schlichting, H. E. & Young, E. D. Chemical equilibrium between cores, mantles, and atmospheres of super-Earths and sub-Neptunes and implications for their compositions, interiors, and evolution. Planet. Sci. J.3, 127 (2022).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

