How to Select Each Component of CO2 Electrolyzers
- PMID: 41163808
- PMCID: PMC12561210
- DOI: 10.1002/EXP.20240025
How to Select Each Component of CO2 Electrolyzers
Abstract
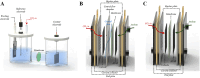
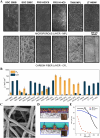
Electrochemical CO2 electrolyzers are increasingly recognized for their potential to convert CO2 into valuable chemical feedstocks, addressing critical environmental and economic challenges. Traditionally, the catalytic properties of the cathode, where CO2RR directly occurs, have been the main focus of research due to their control over product selectivity. More recently, however, membrane-based electrolyzers-commonly used in fuel cells and water electrolyzers-have shown substantial potential for commercial CO2 reduction, offering improved scalability and efficiency. Nevertheless, the complex components in membrane-based electrolyzers require precise optimization, as each unit directly impacts system performance and product selectivity. In this review, the structures and components of membrane-based CO2 electrolyzers are systematically examined, including the electrolyzer design, flow channels, membranes, electrolytes, CO2 supply units, and electrodes. Recent innovations in the optimization of these components are highlighted to provide insights into advancing CO2RR technology toward commercially feasible applications. This approach can assist considerably in improving the CO2RR electrolyzer performance, thereby helping predict optimal pathways for commercial realization and guide future development.
Keywords: CO2 electrolyzer; CO2 reduction reaction; flow channel; membrane; porous transfer electrode.
© 2025 The Author(s). Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Hansen J., Johnson D., Lacis A., et al., “Climate Impact of Increasing Atmospheric Carbon Dioxide,” Science 213 (1981): 957–966. - PubMed
-
- Rodhe H., “A Comparison of the Contribution of Various Gases to the Greenhouse Effect,” Science 248 (1990): 1217–1219. - PubMed
-
- Bushuyev O. S., De Luna P., Dinh C. T., et al., “What Should We Make With CO2 and How Can We Make It?,” Joule 2 (2018): 825–832.
-
- De Luna P., Hahn C., Higgins D., Jaffer S. A., Jaramillo T. F., and Sargent E. H., “What Would It Take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes?,” Science 364 (2019): eeav3506. - PubMed
-
- Le Quéré C., Peters G. P., Friedlingstein P., et al. Nature Climate 11 (2021): 197.
Publication types
LinkOut - more resources
Full Text Sources
