Combining Network Pharmacology, Molecular Docking, and Integrative Studies to Explore the Mechanism of Helminthostachys zeylanica in Alleviating Ulcerative Colitis
- PMID: 41164267
- PMCID: PMC12560106
- DOI: 10.1002/fsn3.71139
Combining Network Pharmacology, Molecular Docking, and Integrative Studies to Explore the Mechanism of Helminthostachys zeylanica in Alleviating Ulcerative Colitis
Abstract
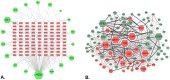
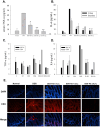
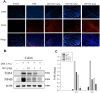
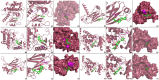
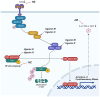
Helminthostachys zeylanica (L.) hook (HZ), has recently gained attention as a potential herbal supplement for managing ulcerative colitis (UC) through its bioactive compounds. To comprehensively investigate HZ's therapeutic effects and underlying mechanisms on UC, we utilized network pharmacology and in vitro and in vivo analyses. The therapeutic potential of HZ was evaluated using a DSS-induced mouse model of ulcerative colitis, alongside in vitro cellular studies. A network pharmacology approach was first used to predict the active compounds and molecular targets of HZ. Subsequently, integrated experimental techniques-including ELISA, Western blotting, histological analysis, immunofluorescence, flow cytometry, and molecular docking-were employed to validate and support the predicted mechanisms. Network pharmacology analysis identified 15 active compounds in HZ, contributing to its multi-target synergistic activity and anti-inflammatory effects. HZ was found to modulate multiple inflammatory pathways, particularly the Toll-like receptor 4 (TLR4) and NF-κB signaling pathways, regulating vital inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), emphasizing its therapeutic potential in UC. ELISA, Western blot, and histological analyses confirmed that HZ significantly reduced colon inflammation. Immunofluorescence and flow cytometry analyses also demonstrated that HZ alleviated inflammation by regulating TLR4/NF-κB and CD3 signaling pathways without involving apoptosis. Ultimately, molecular docking further identified core compounds, including Ugonin M, O, K, and R, which exhibited strong binding affinity to critical proteins in the TLR4/NF-κB pathway, such as TAK1, IKKβ, and RELA, underscoring their role in HZ's anti-inflammatory mechanisms. Collectively, these findings provide a solid basis for further investigation into the mechanistic effects and broader clinical potential of HZ as a therapeutic approach for UC.
Keywords: Helminthostachys zeylanica; TLR4/NF‐κB signaling pathway; network pharmacology; ugonin; ulcerative colitis.
© 2025 The Author(s). Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Abreu‐Martin, M. T. , and Targan S. R.. 1996. “Chapter 18—Lamina Propria Lymphocytes: A Unique Population of Mucosal Lymphocytes.” In Essentials of Mucosal Immunology, edited by Kagnoff M. F. and Kiyono H., 227–245. Academic Press. 10.1016/B978-012394330-9/50020-X. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

