Intranasal Dexmedetomidine with Propofol Provides Superior Sedation for Pediatric Contrast-Enhanced CT: A Randomized Controlled Trial
- PMID: 41164404
- PMCID: PMC12560646
- DOI: 10.2147/DDDT.S544737
Intranasal Dexmedetomidine with Propofol Provides Superior Sedation for Pediatric Contrast-Enhanced CT: A Randomized Controlled Trial
Abstract
Background: Effective and safe sedation with rapid recovery remains a critical unmet need for pediatric patients undergoing contrast-enhanced computed tomography (contrast-enhanced CT). We compared the efficacy of intranasal dexmedetomidine (DEX) combined with intravenous propofol (D-P) versus DEX with buccal midazolam (D-M) for sedation during pediatric contrast-enhanced CT.
Methods: In this single-center, prospective, randomized controlled trial, 110 children (6 months-6 years, ASA I/II) were allocated to D-M (2 μg/kg intranasal DEX + 0.2 mg/kg buccal midazolam) or D-P (2 μg/kg intranasal DEX + 1 mg/kg intravenous propofol). Primary outcome was one-time success rate (completed contrast-enhanced CT without additional sedation). Secondary outcomes included onset time, recovery metrics (Ramsay Sedation Scale [RSS] at 30 minutes, time to oral intake), and adverse events. Analyses followed full-analysis-set (FAS) and per-protocol-set (PPS) principles (ChiCTR2300067469).
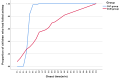
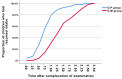
Results: The D-P group demonstrated superior one-time success rates in both FAS (96.4% vs 74.5%; OR 9.05, 95% CI 1.95-42.05, P =0.001) and PPS analyses (96.4% vs 77.1%; OR 7.88, 95% CI 1.65-37.6, P =0.003). Sedation onset was faster with D-P (median 17 vs 20 minutes, P < 0.001), with 98.2% achieving sleep within 20 minutes versus 54.5% for D-M. Recovery was accelerated in D-P: 61.8% attained RSS ≤3 by 30 minutes (vs 30.9%, P < 0.001), and 77.3% resumed oral intake within 1 hour (vs 25.4%, P < 0.001). Bradycardia occurred more frequently with D-P (29.1% vs 5.4%, P =0.001), but no interventions were required.
Conclusion: Intranasal dexmedetomidine combined with propofol significantly improves sedation success, accelerates recovery, and reduces procedural delays in pediatric contrast-enhanced CT compared to midazolam, offering a clinically advantageous regimen for short-duration imaging.
Keywords: computed tomography; dexmedetomidine; midazolam; pediatrics; propofol; sedation.
© 2025 Zhang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Masui T, Katayama M, Kobayashi S, Sakahara H. Intravenous injection of high and medium concentrations of computed tomography contrast media and related heat sensation, local pain, and adverse reactions. J Comput Assist Tomogr. 2005;29(5):704–708. doi: 10.1097/01.rct.0000171238.10678.54 - DOI - PubMed
-
- Gu HB, Miao LY, Bai J, Lu GL, Lei Q, Yang LJ, Wang DG. Combined use of intranasal Dexmedetomidine and an oral novel formulation of Midazolam for sedation of young children during brain MRI examination: a prospective, single-center, randomized controlled trial. BMC Anesthesiol. 2022;22(1):357. doi: 10.1186/s12871-022-01897-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous