Dosimetric and hematological toxicity analyses of bone marrow-sparing intensity-modulated radiation therapy for patients with cervical cancer treated with extended-field radiation therapy
- PMID: 41164422
- PMCID: PMC12559915
- DOI: 10.1002/pro6.70019
Dosimetric and hematological toxicity analyses of bone marrow-sparing intensity-modulated radiation therapy for patients with cervical cancer treated with extended-field radiation therapy
Abstract
Objective: This study aimed to assess the dosimetric parameters and hematological toxicity (HT) associated with bone marrow-sparing (BMS) intensity-modulated radiation therapy (IMRT) in patients diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC cervical cancer undergoing extended-field radiation therapy (EFRT).
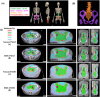
Methods: Patients with cervical cancer presenting with common iliac or para-aortic lymph node metastases require EFRT, which often results in grade 3 HT. Therefore, we retrospectively analyzed data of 84 patients with FIGO stage IIIC cervical cancer who underwent concurrent chemoradiotherapy (EFRT, brachytherapy, and weekly cisplatin 40 mg/m2) at Harbin Medical University Cancer Hospital, including 40 who received BMS-IMRT and 44 who received normal IMRT. Dose-volume histogram (DVH) parameters and estimated treatment times were compared. We also compared acute HT between the normal and BMS groups.
Results: Dosimetric analysis demonstrated that BMS-IMRT significantly reduced the mean volume of bone marrow receiving ≥10, ≥20, ≥30, and ≥40 Gy without affecting the target coverage of planning target volume and sparing the organs at risk. Within the BMS-IMRT group, 37.5% of the patients developed grade ≥3 HT, with an increase in HT (HT3+ = 61.4%) in patients receiving normal-IMRT (P = 0.029).
Conclusions: For patients with cervical cancer treated with EFRT, BMS-IMRT represents a feasible treatment approach that may mitigate HT and facilitate the uninterrupted administration of concurrent chemoradiotherapy.
Keywords: bone marrow sparing; cervical cancer; extended‐field radiation therapy; hematological toxicity; intensity‐modulated radiation therapy.
© 2025 The Author(s). Precision Radiation Oncology published by John Wiley & Sons Australia, Ltd on behalf of Shandong Cancer Hospital & Institute.
Conflict of interest statement
All authors declare no potential conflicts of interest.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Rose PG, Java J, Whitney CW, et al. Nomograms predicting progression‐free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG oncology/gynecologic oncology group randomized trials of chemoradiotherapy. J Clin Oncology. 2015;33(19):2136‐2142. - PMC - PubMed
-
- Mohamud A, Høgdall C, Schnack T. Prognostic value of the 2018 FIGO staging system for cervical cancer. Gynecol Oncol. 2022;165(3):506‐513. - PubMed
-
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. The Lancet. 2019;393(10167):169‐182. - PubMed
LinkOut - more resources
Full Text Sources
