Dissociation of the nuclear basket triggers chromosome loss in aging yeast
- PMID: 41165159
- PMCID: PMC12574999
- DOI: 10.7554/eLife.104530
Dissociation of the nuclear basket triggers chromosome loss in aging yeast
Abstract
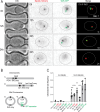
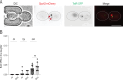
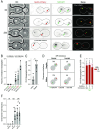
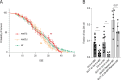
In many organisms, aging is a clear risk factor for chromosome missegregation, the main source of aneuploidy. Here, we report that old yeast cells lose chromosomes by partitioning them asymmetrically to their daughter cells together with the pre-existing (old) spindle pole body (SPB, centrosome equivalent in yeast). Strikingly, remodelling of the nuclear pore complex (NPC) and the displacement of its nuclear basket triggered these asymmetric chromosome segregation events. Simultaneously, nuclear basket displacement caused unspliced pre-mRNAs to leak into the cytoplasm. We show that removing the introns of three genes involved in chromosome segregation was sufficient to fully suppress chromosome loss in old cells. Promoting pre-mRNA leakage in young cells also caused asymmetric chromosome partitioning and loss through the same three introns. Therefore, we propose that basket displacement from NPCs and its consequences for pre-mRNA quality control are key triggers of aging phenotypes such as aneuploidy.
Keywords: NPC; RNA; S. cerevisiae; aging; aneuploidy; cell biology; chromosomes; gene expression; intron; mitosis.
© 2025, Mirkovic et al.
Conflict of interest statement
MM, JM, AM, JP, SL, SE, YB No competing interests declared
Figures





Update of
- doi: 10.1101/2024.10.31.621394
- doi: 10.7554/eLife.104530.1
- doi: 10.7554/eLife.104530.2
Comment in
- doi: 10.7554/eLife.109320
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

