Long-term outcomes of elosulfase alfa enzyme replacement therapy in adults with MPS IVA: a sub-analysis of the Morquio A Registry Study (MARS)
- PMID: 41168830
- PMCID: PMC12574230
- DOI: 10.1186/s13023-025-04064-w
Long-term outcomes of elosulfase alfa enzyme replacement therapy in adults with MPS IVA: a sub-analysis of the Morquio A Registry Study (MARS)
Abstract
Background: Mucopolysaccharidosis (MPS) IVA is a rare disease with substantial, multisystemic morbidity. We assessed real-world safety and effectiveness of the enzyme replacement therapy (ERT) elosulfase alfa in patients with MPS IVA in the multinational, observational Morquio A Registry Study (MARS) who initiated ERT in adulthood (aged ≥ 18 years).
Methods: Patients were enrolled between September 2014 and February 2022; urinary keratan sulfate (uKS), 6-minute walk test (6MWT) distance, forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), EuroQoL-5D-5L (EQ-5D-5L) score, and safety were assessed during routine care.
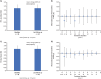
Results: As of February 13, 2022, 90 patients who initiated ERT had enrolled (median exposure: 5.6 years; median age at first ERT: 27.8 years). Reductions from baseline in uKS levels were sustained over mean follow-up of 5.4 years (mean percent change: -52.9%; p < 0.0001). In patients with available data, mean change in 6MWT distance was + 15.8 m (p = 0.3627) over a mean follow-up of 5.8 years. FEV1 and FVC remained stable over mean follow-up of 5.3 years (mean change: 0.0 L for both). The mean change from baseline in EQ-5D-5L index score was + 0.1 after 1 year of treatment. Thirty-four patients (39.5%) had ≥ 1 adverse event (AE), 23 patients (26.7%) had ≥ 1 serious AE, and 10 (11.6%) had ≥ 1 drug-related AE (infusion-related reactions [n = 3; 3.5%], pyrexia [n = 2; 2.3%]). Eight deaths occurred; none were deemed treatment related.
Conclusions: Real-world data collected from MARS suggest that patients with MPS IVA who initiated ERT in adulthood remained stable over 7 years of follow-up. No new safety signals were identified.
Keywords: Clinical outcomes; Elosulfase alfa; Mucopolysaccharidosis IVA; Patient-reported outcomes; Real-world evidence.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approvals and consent for participation: The MARS study protocol was reviewed and approved by all applicable institutional review boards and ethics committees for participating study sites. All participants gave written informed consent, or, in the case of individuals aged < 18 years, written consent was obtained from a legally authorized representative. All publications related to the MARS registry have been overseen by a dedicated Scientific Publication Committee. Consent for publication: Not applicable. Competing interests: KMS is a Principal Investigator in MARS for BioMarin Pharmaceutical Inc. BKB has received consulting payments from Aeglea, Agios, Altrna, Aro, BioMarin Pharmaceutical Inc., JCR Pharma, Jnana, Maze, Moderna, Passage Bio, PTC Therapeutics, Takeda, and Travere; has participated as a clinical trial investigator for BioMarin Pharmaceutical Inc., Denali, JCR Pharma, Sangamo, and Ultragenyx; and has received speaker fees/payments from BioMarin Pharmaceutical Inc., Chiesi, Horizon, Takeda, and Ultragenyx. MBB is an employee and owner of Tyra Biosciences; and has received consulting payments from and participated as a clinical trial investigator for BioMarin Pharmaceutical Inc. PMC has provided consultation/advisory services for BioMarin Pharmaceutical Inc., Ipsen, and Sanofi. CE has received honorarium for participation in a MARS Medical Advisory Board from BioMarin Pharmaceutical Inc. KB has nothing to report. NG has received consulting payments and travel support from and participated as a clinical trial investigator in MARS for BioMarin Pharmaceutical Inc.; is a Principal Investigator in clinical trials for Chiesi, JCR Pharma, Sanofi, Takeda, and Ultragenyx; and has received consulting/speaker fees from Chiesi, JCR Pharma, Sanofi, and Ultragenyx. DH, AH, and PR are employees of and may hold stock ownership and shareholdings in BioMarin Pharmaceutical Inc. AL has nothing to report. S-PL has provided advisory board services to BioMarin Pharmaceutical Inc. MM has received lecture honoraria and consultancy fees from BioMarin Pharmaceutical Inc., Chiesi, and Takeda. EM has participated as a clinical trial investigator for BioMarin Pharmaceutical Inc. JJM has received consulting payments from BioMarin Pharmaceutical Inc., Denali, Sanofi-Genzyme, Takeda, and Ultragenyx; has received research grants from BioMarin Pharmaceutical Inc., Sanofi-Genzyme, and Takeda; and has participated as a clinical trial investigator in MARS for BioMarin Pharmaceutical Inc.
Figures



References
-
- Montaño AM, Tomatsu S, Gottesman G, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30(2):165–74. - PubMed
-
- Harmatz P, Mengel KE, Giugliani R, Valayannopoulos V, Lin SP, Parini R, et al. The Morquio A Clinical Assessment Program: baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol Genet Metab. 2013;109(1):54–61. - PubMed
-
- Harmatz PR, Mengel KE, Giugliani R, Valayannopoulos V, Lin S-P, Parini R, et al. Longitudinal analysis of endurance and respiratory function from a natural history study of Morquio A syndrome. Mol Genet Metab. 2015;114(2):186–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

