Human indels as predictors of antibody responses to COVID-19 vaccines
- PMID: 41169506
- PMCID: PMC12570367
- DOI: 10.1016/j.isci.2025.113475
Human indels as predictors of antibody responses to COVID-19 vaccines
Abstract
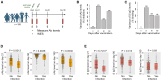
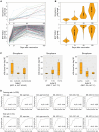
Vaccine efficacy varies significantly among adults. This variability underlies the limitation of a one-size-fits-all vaccination strategy and the need for more personalized approaches. We investigated factors influencing inter-individual variability in antibody responses to COVID-19 mRNA vaccine among adults. Neutralizing antibody (nAb) levels after the first vaccine dose were associated with infection outcomes within 1 year after vaccination, suggesting their potential as a correlate of protection. Age, sex, and Chinese ethnicity were associated with nAb and anti-spike protein antibody levels. Two indels located at chr1:31433042 and chr15:76311269 showed significant association with antibody responses. Leveraging these host factors, we developed a Random Forest model that predicted vaccine-induced antibody responses with 72.7% accuracy for mRNA vaccine and 76.9% for the Sinopharm COVID-19 inactivated virus vaccine. These findings support predictive modeling as a tool to identify individuals at risk of low vaccine responses, enabling more targeted and effective vaccination strategies.
Keywords: Public health; Virology.
© 2025 The Authors.
Conflict of interest statement
A patent application has been filed (Singapore patent#10202400609T, #10202403182P, PCT/SG2025/050121: Genetic Signatures For Predicting Vaccine Response and Uses Thereof) (H.V.C., L.F.P.N., L.R., Y.S.G., S.W.F., and M.Z.T.).
Figures



References
LinkOut - more resources
Full Text Sources

