Brainstem neurochemical profiles after hospitalisation for COVID-19: a 7T MR spectroscopy study
- PMID: 41169742
- PMCID: PMC12568683
- DOI: 10.3389/fnins.2025.1617709
Brainstem neurochemical profiles after hospitalisation for COVID-19: a 7T MR spectroscopy study
Abstract
Background: Somatic, cognitive and mental health issues have been identified in three-quarters of people 5 months after hospitalisation for severe acute SARS-CoV-2 (COVID-19) infection. The underlying neuroanatomical basis of these symptoms remains unclear, but recent studies suggest a role for altered brainstem physiology. We aimed to test the hypothesis that brainstem neurochemical profiles differ in patients who had been hospitalised for COVID-19 compared to matched controls using 7T magnetic resonance spectroscopy (MRS).
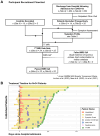
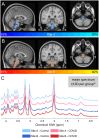
Methods: This prospective case-control study recruited 34 individuals who were hospitalised for COVID-19 and 15 healthy controls with no history of COVID-19 infection from two major UK hospitals before vaccines became available. The participants underwent 7T semi-adiabatic localization by adiabatic selective refocusing (sLASER) 1H-MRS at the ponto-medullary junction. Water-referenced metabolite concentrations were compared between the patients and controls and correlated with infection severity, as measured by maximum C-reactive protein (CRPmax) assay during inpatient admission. Linear mixed modelling was used with a 0.05 significance level.
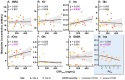
Results: Spectral quality was high/acceptable in 44/49 participants according to the MRS Consensus criteria. The magnitude of inflammation during patient admission (i.e., CRPmax) correlated positively with myo-inositol concentration (β = 0.005, p = 0.035), as did patient-reported symptoms (β = -0.564, p = 0.023). However, metabolite concentrations were not significantly different between the patients and controls.
Conclusion: We show the feasibility of assessing brainstem neurochemical profiles using 7T 1H-MRS in a multi-centre study. Technical limitations at one site's 7T MRI led to variable repetition times, which limited our statistical power and should be avoided in future studies. Our findings highlight the need for further investigation into the role of neuroinflammation in post-acute COVID-19.
Keywords: 7T; COVID-19; brainstem; magnetic resonance spectroscopy; neuroinflammation.
Copyright © 2025 Graf, Raman, Manktelow, Chatfield, Clarke, Rua, Newcombe, Lupson, Sawcer, Outtrim, Ersche, Qiu, Ezra, McDonald, Clare, Cassar, Neubauer, Bullmore, Menon, Rowe, Pattinson and Rodgers.
Conflict of interest statement
VN holds grants from Roche Pharmaceuticals for an analysis outside the presented work. KE receives editorial honoraria from Karger Publishers and is a Trustee for the Society for the Study of Addiction. KP is named as a co-inventor on a provisional UK patent titled “Discordant sensory stimulus in VR based exercise” UK Patent office application: 2204698.1 filing date 31/3/2022. ChR receives research funding from Siemens Healthcare for a different project. The author(s) declared BR was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

