The Implementation of Faricimab in East Lancashire NHS Trust: Highlighting the Real-World Efficacy and Safety in Patients With Neovascular Age-Related Macular Degeneration
- PMID: 41170269
- PMCID: PMC12570282
- DOI: 10.7759/cureus.93478
The Implementation of Faricimab in East Lancashire NHS Trust: Highlighting the Real-World Efficacy and Safety in Patients With Neovascular Age-Related Macular Degeneration
Abstract
Purpose: To report the safety and efficacy of intravitreal faricimab injections (IVTF) in both treatment-naïve and treatment-resistant patients with neovascular age-related macular degeneration (nAMD).
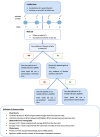
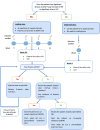
Patients and methods: This study was a retrospective real-world evidence trial where patients with nAMD were given IVTF. Group 1 (treatment-naïve) and group 2 (switch loading dose, SLD) were given a course of four loading doses of IVTF four weeks apart. Group 3 (switch pro re nata, SPRN) was given a single IVTF and then reviewed at eight weeks. The outcome was based on visual acuity (VA) and optical coherence tomography (OCT), showing central retinal thickness (CRT), subretinal, and intraretinal fluid.
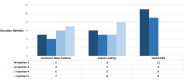
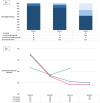
Results: A total of 99 eyes from 89 patients were included. A total of 350 IVTF were given. The mean follow-up duration was 6.33 ± 1.11 months (range: 2-8 months). The mean change in CRT at final follow-up was -127.09 ± 97.39 µm (p < 0.001) for naïve eyes, -115.59 ± 145.02 µm (p < 0.001) for SLD, and -57.61 ± 58.06 µm (p < 0.001) for SPRN. The mean VA change was -3.47 ETDRS (Early Treatment Diabetic Retinopathy Study) (p = 0.256) for naïve eyes, -0.79 (p = 0.45) for SLD, and +1.25 (p = 0.304) for SPRN. A total of 48 eyes (48.48%) had better VA, 22 (22.22%) had no change in VA, and 29 (29.29%) had worse VA. One patient developed bilateral sterile intraocular inflammation.
Conclusion: IVTF was associated with an improvement in CRT in both treatment-naive and treatment-resistant nAMD patients. There was no evidence of vasculitis or vein occlusion, highlighting the safety of faricimab in our real-world study.
Keywords: faricimab; intravitreal (ivt) faricimab; neovascular age-related macular degeneration; neovascular age-related macular disorders; real-world evidence.
Copyright © 2025, Lloyd et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. East Lancashire Hospitals NHS Trust (ELHT) Ethics Committee issued approval N/A. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Salwa Abugreen declare(s) non-financial support and N/A from Novartis, Bayer, and Roche. Salwa Abugreen is an advisory board member of Novartis, Bayer, and Roche. She has been invited to speak at national Novartis and Roche seminars. She has received a travel grant in the past from Novartis, Bayer, and Roche. No other authors have any other conflicts of interest to note. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- A consensus on risk mitigation for brolucizumab in neovascular age-related macular degeneration: patient selection, evaluation, and treatment. Holz FG, Iida T, Maruko I, Sadda SR. https://pubmed.ncbi.nlm.nih.gov/35994582/ Retina. 2022;42:1629–1637. - PMC - PubMed
-
- Action on neovascular age-related macular degeneration (nAMD): recommendations for management and service provision in the UK hospital eye service. Gale RP, Mahmood S, Devonport H, et al. https://www.nature.com/articles/s41433-018-0300-3 Eye (Lond) 2019;33:1–21. - PMC - PubMed
-
- NICE. Faricimab for treating wet age-related macular degeneration. Technology appraisal guidance. Reference number: TA800. 2023. https://www.nice.org.uk/guidance/ta800 https://www.nice.org.uk/guidance/ta800
-
- Spotlight on faricimab in the treatment of wet age-related macular degeneration: design, development and place in therapy. Nair AA, Finn AP, Sternberg P Jr. https://www.tandfonline.com/doi/full/10.2147/DDDT.S368963 Drug Des Devel Ther. 2022;16:3395–3400. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
