Mycobacterium tuberculosis triggers reduced inflammatory cytokine responses and virulence in mice lacking Tax1bp1
- PMID: 41171885
- PMCID: PMC12588459
- DOI: 10.1371/journal.ppat.1012829
Mycobacterium tuberculosis triggers reduced inflammatory cytokine responses and virulence in mice lacking Tax1bp1
Abstract
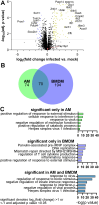
Host responses - autophagy, cell death, and inflammation - limit the growth of bacterial pathogens while minimizing tissue damage. During the early stages of infection, Mycobacterium tuberculosis (Mtb) thwarts these and other innate immune defense mechanisms in alveolar macrophages (AMs) derived from the yolk sac; in later stages, it circumvents defenses in recruited mononuclear cells (MNCs) and survives within them despite additional cytokine stimulation from recruited T cells. The mechanisms that drive variable rates of Mtb growth in different macrophage subtypes and how Mtb manipulates inflammatory responses to grow within innate immune cells remain obscure. Here we explored the role of the host factor, Tax-1 binding protein 1 (Tax1bp1), an autophagy receptor that targets pathogens for degradation through selective autophagy and terminates pro-inflammatory cytokine responses. Unexpectedly, we found that Tax1bp1-deficient mice were less susceptible to Mtb infection, and generated reduced inflammatory cytokine responses, compared to wild-type mice; the same mutant mice exhibited decreased growth of, and inflammatory cytokine responses to, Listeria monocytogenes, suggesting that Tax1bp1 plays a role in host responses to multiple intracellular pathogens. Contrary to our previous ex vivo findings in bone marrow-derived macrophages (BMDMs), in vivo growth of Mtb in AMs and a subset of recruited MNCs was more limited in mice lacking Tax1bp1 relative to wild-type mice. To better understand these differences, we performed global protein abundance measurements in mock- and Mtb-infected AM samples ex vivo from wild-type mice. These experiments revealed that Tax1bp1 protein abundance does not significantly change early after infection in AMs but does in BMDMs; moreover, early after infection, Tax1bp1-deficiency reduced necrotic-like cell death -- an outcome that favors Mtb replication -- in AMs but not BMDMs. Together, these results show that deficiency of Tax1bp1 plays a crucial, cell type-specific role in linking the regulation of autophagy, cell death, and anti-inflammatory host responses and overall reducing bacterial growth.
Copyright: © 2025 Chin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





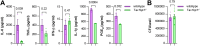

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

