Efficacy of Anlotinib in Treating Progressive Glioblastoma: Insights From a Single-Center Study
- PMID: 41174836
- PMCID: PMC12588388
- DOI: 10.12659/MSM.948367
Efficacy of Anlotinib in Treating Progressive Glioblastoma: Insights From a Single-Center Study
Abstract
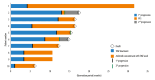
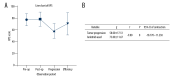
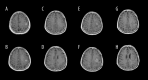
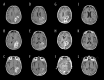
BACKGROUND Glioblastoma (GBM) is the most common and aggressive malignant tumor in the central nervous system, with limited therapeutic options and poor prognosis. Anlotinib, a novel multi-targeted tyrosine kinase inhibitor, has shown promise in treating various malignancies. This study systematically analyzed the treatment outcomes of 10 typical patients with progressive GBM in our institution who were treated with anlotinib. MATERIAL AND METHODS Ten progressive GBM patients treated with anlotinib between 2020 and 2022 were included. Tumor progression was assessed using modified Response Assessment in Neuro-Oncology (mRANO) criteria. Disease progression was evaluated via conventional MRI and physical examinations. Patient condition was measured using the Karnofsky performance status (KPS) scale and the European Organization for Research and Treatment Core Quality of Life Questionnaire (EORTC QLQ-C30). Median progression-free survival (PFS) and overall survival (OS) were calculated from anlotinib initiation. Adverse effects were graded using CTCAE 5.0. RESULTS According to the mRANO criteria, 3 patients had a complete response, 3 had a partial response, 1 had stable disease, and 3 had progressive disease, resulting in a 70% disease control rate and a 60% objective response rate. Median PFS was 5.42 months, and median OS was 6.30 months. KPS scores significantly improved after treatment (P<0.05), and QLQ-C30 scores were higher in 11 of 15 items (P<0.05). No grade 3 or 4 adverse events were observed. CONCLUSIONS Through small-sample real-world research, anlotinib, either as monotherapy or in combination with temozolomide, demonstrated promising therapeutic effects in patients with progressive GBM, suggesting its potential as a targeted treatment option.
Conflict of interest statement
Figures

References
-
- McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ. 2021;374:n1560. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
