Long-term effectiveness and safety of benralizumab in EGPA: a 3-year single-center experience
- PMID: 41178590
- PMCID: PMC12584834
- DOI: 10.1080/07853890.2025.2581812
Long-term effectiveness and safety of benralizumab in EGPA: a 3-year single-center experience
Abstract
Objectives: Benralizumab emerged as a promising treatment option for eosinophilic granulomatosis with polyangiitis (EGPA). This study assessed the long-term effectiveness and safety of benralizumab in patients with severe asthma and relapsing-refractory EGPA.
Methods: This retrospective, single-center study evaluated patients treated with benralizumab (30 mg/8 weeks), followed for up to 36 months. Primary outcome included disease remission (defined as Birmingham Vasculitis Activity Score version 3 = 0 and prednisone dose ≤4 mg/day). Secondary endpoints were corticosteroid tapering, lung function, relapses, treatment failure and drug retention rates.
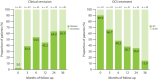
Results: The study included 33 EGPA patients (17 [51.5%] male; median age at benralizumab initiation 56 years [IQR: 47-62]). Before starting benralizumab, most patients were on corticosteroids (90.9%), prior treatments included mepolizumab (24.2%). Benralizumab showed effectiveness, with clinical remission rates increasing from 39.4% (95% CI: 22.9-57.9) at 3 months to 65.0% (95% CI: 40.8-84.6) at 36 months (p < 0.001). Corticosteroid use declined from 90.9% to 15.4%, eosinophil counts dropped from 850 (515-1367) to 0 (0-0) cells/µL, and BVASv3 decreased from 2 (2-5) to 0 (0-0), showing significant improvements (p = 0.002 and p < 0.001, respectively). Proportion of patients experiencing asthma exacerbations reduced, alongside improved lung function. Retention rates were 81.8% at 1 year, 72.6% at 2 years, and 62.4% at 3 years, with secondary failure due to uncontrolled sinonasal symptoms. Mild adverse events were observed in 21.2% of patients.
Conclusions: These findings support the long-term effectiveness and safety of benralizumab for EGPA, highlighting its role in inducing clinical remission, reducing corticosteroid dependence, and controlling disease activity.
Keywords: Asthma; Benralizumab; Eosinophilic granulomatosis with polyangiitis; Vasculitis.
Conflict of interest statement
RP reports being invited as a speaker or advisory board member by GSK, AstraZeneca, Sanofi, and CSL Vifor outside the current work. AV received research grants from CLS Behring, GSK, and AstraZeneca. All other authors declare they have no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
