Pan-immune-inflammation value as a novel predictor of pathological response to neoadjuvant chemotherapy combined with anti-PD-1 therapy in esophageal squamous cell carcinoma: a multicenter real-world retrospective clinical study
- PMID: 41179120
- PMCID: PMC12579197
- DOI: 10.1177/17588359251378883
Pan-immune-inflammation value as a novel predictor of pathological response to neoadjuvant chemotherapy combined with anti-PD-1 therapy in esophageal squamous cell carcinoma: a multicenter real-world retrospective clinical study
Abstract
Background: Current biomarkers for predicting pathological response to neoadjuvant chemoimmunotherapy in esophageal squamous cell carcinoma (ESCC) remain limited.
Objective: This study investigates the potential of the pan-immune-inflammation value (PIV) as a biomarker for predicting pathological response after neoadjuvant chemoradiotherapy combined with anti-programmed death protein-1 (PD-1) therapy in ESCC.
Design: A multicenter, real-world, retrospective clinical study conducted across five centers in Southern China (January 2021-March 2024).
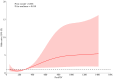
Methods: A multicenter retrospective study included 334 patients with ESCC, divided into pathological complete response (pCR) and non-pathological complete response (non-pCR) groups. Clinical and laboratory data were analyzed using univariate and multivariate logistic regression to evaluate the relationship between post-treatment PIV and pathological response. The threshold effect of PIV was explored using restricted cubic spline analysis.
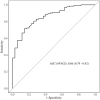
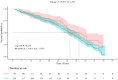
Results: Subgroup analysis showed no significant interactions across clinical subgroups. Post-treatment PIV was positively associated with non-pCR risk (odds ratio = 1.002; 95% confidence interval: 1.001-1.003, p < 0.005). A positive association was observed in the high-PIV stratum (⩾280), where elevated PIV levels significantly correlated with increased non-pCR risk. Receiver operating characteristic analysis showed an area under the curve of 0.86 for predicting non-pCR, with a sensitivity of 86.6% and specificity of 72% at an optimal cutoff of 438.04. The high-PIV group exhibited inferior survival outcomes with significantly increased mortality risk.
Conclusion: Post-treatment PIV shows a nonlinear relationship with pathological response in patients receiving neoadjuvant chemoradiotherapy combined with anti-PD-1 therapy and may serve as a predictive biomarker.
Keywords: esophageal squamous cell carcinoma; inflammatory markers; neoadjuvant chemotherapy; pathological response; robotic-assisted.
© The Author(s), 2025.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. - PubMed
-
- Yang W, Liu F, Xu R, et al. Is adjuvant therapy a better option for esophageal squamous cell carcinoma patients treated with esophagectomy? A prognosis prediction model based on multicenter real-world data. Ann Surg 2023; 277(1): e61–e69. - PubMed
-
- Guo X, Wang Z, Yang H, et al. Impact of lymph node dissection on survival after neoadjuvant chemoradiotherapy for locally advanced esophageal squamous cell carcinoma: from the results of NEOCRTEC5010, a randomized multicenter study. Ann Surg 2023; 277(2): 259–266. - PubMed
-
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(11): 1506–1517. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

