CsUGT89A2 enhances tea plant resistance to Toxoptera aurantia by mediating flavonoid glycosides biosynthesis
- PMID: 41180024
- PMCID: PMC12577854
- DOI: 10.1093/hr/uhaf212
CsUGT89A2 enhances tea plant resistance to Toxoptera aurantia by mediating flavonoid glycosides biosynthesis
Abstract
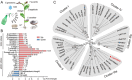
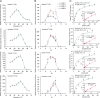
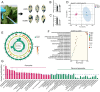
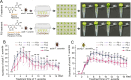
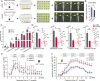
Tea plant [Camellia sinensis (L.) O. Kuntze] is a globally important crop but is severely threatened by Toxoptera aurantia infestations, which impact yield and safety. However, the response of tea plants to aphid feeding remains largely unexplored. This study investigates the feeding behavior of T. aurantia on different cultivars and identifies 'Huangjinya' and 'Qiancha 1' as susceptible and resistant cultivars, respectively. Transcriptome analysis revealed that CsUGT89A2 was significantly upregulated in response to T. aurantia infestation. In vitro biochemical assays demonstrated that CsUGT89A2 encodes a flavonoid 7-glycosyltransferase that catalyzes the conversion of flavonoids and UDP-glucose into flavonoid 7-O-glucosides. In vivo, silencing CsUGT89A2 significantly reduced flavonoid glycoside accumulation. To further clarify the role of CsUGT89A2 in tea plant resistance to T. aurantia, we used tobacco and tea flowers to evaluate aphid feeding and reproduction under chemical treatment, gene silencing, and gene overexpression conditions. Statistical analysis showed that, compared with flavonoids, the application of flavonoid 7-O-glycosides significantly reduced T. aurantia reproductive capacity. Furthermore, compared with the control, overexpression of CsUGT89A2 significantly reduced the reproductive ability of aphids, while its silencing increased reproductive rates. Overall, our findings demonstrate that CsUGT89A2 mediates flavonoid glycosylation and enhances insect resistance in tea plants by increasing flavonoid glycoside levels, offering new insights into the role of flavonoid glycosides in the insect resistance of C. sinensis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Nanjing Agricultural University.
Conflict of interest statement
The authors declare that there are no conflicts of interest between them.
Figures





References
LinkOut - more resources
Full Text Sources

