Water-soluble cationic porphyrins with enhanced phototoxicity to cancer cell lines for G4-targeting photodynamic therapy
- PMID: 41180470
- PMCID: PMC12577588
- DOI: 10.1039/d5md00706b
Water-soluble cationic porphyrins with enhanced phototoxicity to cancer cell lines for G4-targeting photodynamic therapy
Abstract
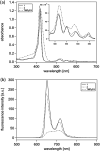
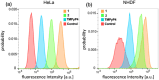
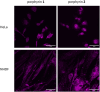
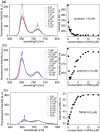
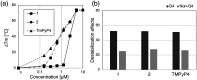
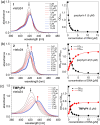
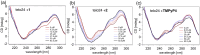
Porphyrins are well-known photosensitizers (PSs), a few of which are clinically approved drugs for use in photodynamic therapy (PDT). Porphyrin derivatives including tetra-cationic porphyrins, e.g.TMPyP4, are also well-studied binders for G-quadruplex (G4) DNA. Since G4 DNAs are known to play a role in malignant transformation of cells, a variety of G4 binders have been used in cancer therapy by regulating the function of G4 DNA. In this study, two water-soluble porphyrins (1 and 2), with four terminal cationic moieties connected with alkyl linkers were synthesized as bifunctional molecules for simultaneous G4 binding and PDT-PS. Photoinduced singlet oxygen (1O2) generation and DNA cleavage were tested under visible light irradiation revealing the efficient generation of 1O2 in line with photoinduced DNA cleavages. Studies in a cancer cell line (HeLa) and a normal fibroblast (NHDF) cells revealed significantly stronger photocytotoxicities of these porphyrins (1 and 2) in comparison to TMPyP4, presumably due to better cellular internalization - as observed by flow cytometry. Interestingly, enhanced photocytotoxicity of 1 and 2 was observed in HeLa in comparison to NHDF. This may be related to the fact that more G4 DNAs are present in the nuclei of cancer cell lines to allow binding of porphyrins 1 and 2, as observed by fluorescence microscopy. The interactions of porphyrins 1 or 2 with a G4-forming telomeric DNA were evaluated by a FRET assay and spectroscopic methods (fluorescence, UV-vis, and CD) and showed selective binding to G4 DNA. The results show the potential of porphyrins 1 and 2 as PDT-PSs targeting cancer cells with higher G4-forming domains.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Lovell J. F. Liu T. W. B. Chen J. Zheng G. Chem. Rev. 2010;110:2839–2857. - PubMed
-
- Pandey R. K. and Zheng G., The Porphyrin Handbook, 2000, vol. 6, pp. 157–230
-
- Nakajima S. Hayashi H. Omote Y. Yamazaki Y. Hirata S. Maeda T. Kubo Y. Takemura T. Kakiuchi Y. Shindo Y. Koshimizu K. Sakata I. J. Photochem. Photobiol., B. 1990;7:189–198. - PubMed
-
- Hiyama K. Matsui H. Tamura M. Shimokawa O. Hiyama M. Kaneko T. Nagano Y. Hyodo I. Tanaka J. Miwa Y. Ogawa T. Nakanishi T. Tamai I. J. Porphyrins Phthalocyanines. 2013;17:36–43.
LinkOut - more resources
Full Text Sources

