A20 mRNA therapeutics ameliorate systemic sclerosis by suppressing TRAF-6/NF-KB signaling and DREAM expression and exerting antifibrotic effects
- PMID: 41181125
- PMCID: PMC12571734
- DOI: 10.3389/fimmu.2025.1665998
A20 mRNA therapeutics ameliorate systemic sclerosis by suppressing TRAF-6/NF-KB signaling and DREAM expression and exerting antifibrotic effects
Abstract
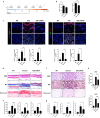
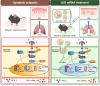
Introduction: Systemic sclerosis (SSc) is a chronic autoimmune disorder characterized by progressive fibrosis, vascular abnormalities, and immune dysregulation. Decreased expression of A20 (TNFAIP3), a key negative regulator of inflammation, has been shown to aggravate SSc pathogenesis by enhancing fibroblast activation and promoting collagen production. This study explores the therapeutic efficacy of A20 mRNA-lipid nanoparticle (LNP) delivery in restoring A20 expression and mitigating fibrosis through modulation of the TRAF6/NF-κB and Downstream Regulatory Element Antagonist Modulator (DREAM) -SMAD2 signaling pathways.
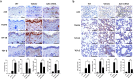
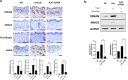
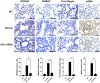
Methods: Human dermal fibroblasts were transfected with A20 mRNA -LNP and subsequently stimulated with transforming growth factor-beta (TGF-β). Protein expression levels and fibrotic markers were analyzed by Western blotting and quantitative PCR. In vivo, a bleomycin-induced mouse model of SSc received weekly intramuscular injections of A20 mRNA -LNP. The extent of fibrosis was assessed through histological analysis and immunohistochemistry.
Results: Transfection with A20 mRNA-LNP significantly suppressed TRAF6/NF-κB signaling and reduced fibrotic marker expression in vitro. In the SSc mouse model, A20 mRNA-LNP treatment markedly attenuated skin and lung fibrosis and decreased collagen deposition. Importantly, A20 overexpression led to downregulation of DREAM in vivo and inhibition of SMAD2 phosphorylation in vitro, indicating crosstalk between inflammatory and fibrotic pathways.
Discussion: A20 mRNA therapy effectively alleviates fibrosis by restoring A20 expression and inhibiting TRAF6/NF-κB signaling, while also downregulating DREAM, a previously unrecognized target. This dual-pathway regulation underscores the role of A20 and DREAM as central modulators of fibrotic progression. These findings highlight the potential of A20 mRNA-LNP as a novel therapeutic strategy for SSc, offering a multifaceted approach that may surpass current treatment options by simultaneously targeting interconnected pathogenic pathways.
Keywords: A20 (TNFAIP3); dream; fibrosis; immune cells; systemic sclerosis.
Copyright © 2025 Lee, Jeon, Kwak, Yang, Bang, Lee, Lee, Park, Han, Choi, Park, Cho and Nam.
Conflict of interest statement
Authors HWK, YJB and J-HN were employed by company SML Biopharm. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

