Comprehensive profiling of host- and virus-derived circular RNAs during vesicular stomatitis virus infection
- PMID: 41181322
- PMCID: PMC12571801
- DOI: 10.3389/fcimb.2025.1654185
Comprehensive profiling of host- and virus-derived circular RNAs during vesicular stomatitis virus infection
Abstract
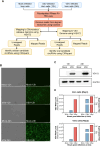
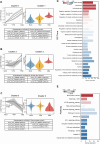
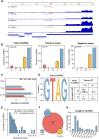
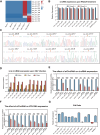
Circular RNA (circRNA) is a new member of noncoding RNA family, which has garnered increasing attention, particularly in the context of viral infections. Vesicular stomatitis virus (VSV) is a negative-sense RNA virus that threatens animal husbandry and currently lacks effective treatments. Despite extensive studies on VSV in basic research and medical applications, the systemic profiling of circRNAs in the context of VSV remains unexplored. In this study, we conducted a comprehensive analysis of circRNA profiles in VSV-infected Vero cells using high-throughput sequencing. We identified a total of 65,645 host-derived cellular circRNAs, of which 1,682 were differentially expressed. Trend clustering revealed three significant expression patterns, and functional annotation indicated that cluster 1 was associated with proviral pathways. Subsequent results showed that VSV infection elevated the top 10 cellular circRNAs, which in turn promoted VSV replication. Additionally, we identified 120 virus-derived circRNAs, top 10 of which were upregulated by VSV and enhanced VSV infection as well. We also characterized the general features of both cellular and viral circRNAs, including genomic locations and back-splicing signals. In summary, our findings revealed that both host cellular and viral circRNAs are induced by VSV infection, subsequently affecting VSV infection. This study unveils a previously unrecognized layer of virus-host interactions involving circRNAs, which may assist in the development of control strategies for VSV and its fundamental and medical applications.
Keywords: RNA-seq2; cellular circRNAs3; differentially expressed circRNAs5; vesicular stomatitis virus1; viral circRNAs4.
Copyright © 2025 Miao, Mai, Zhu, Lin, Yang, Qiu, Wang, Liu and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

