The miRNA Expression of Urinary Extracellular Vesicles in Patients With Gitelman Syndrome: The Role of hsa-let-7d-3p
- PMID: 41182646
- PMCID: PMC12582352
- DOI: 10.1096/fj.202501394RRR
The miRNA Expression of Urinary Extracellular Vesicles in Patients With Gitelman Syndrome: The Role of hsa-let-7d-3p
Abstract
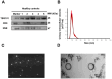
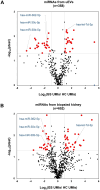
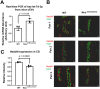
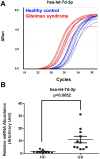
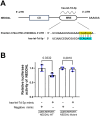
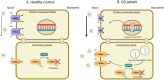
Gitelman syndrome (GS) is caused by an inactivating mutation in the SLC12A3, encoding the thiazide-sensitive sodium chloride cotransporter (NCC), leading to salt wasting and electrolyte imbalance. Our aim is to identify microRNA (miRNA) expression and explore their role from urinary extracellular vesicles (uEVs) in GS patients. In this study, both uEVs from 23 genetically confirmed GS patients and renal biopsied tissues from another 3 GS patients were extracted for small RNA sequencing. Small RNA sequencing identified 358 miRNAs from uEVs and 652 miRNAs from renal biopsied tissues. Among differentially expressed miRNAs, 20 were upregulated and 23 downregulated in uEVs, while renal biopsied tissues showed 30 upregulated and 23 downregulated miRNAs. Four miRNAs (hsa-let-7d-3p, hsa-miR-362-5p, hsa-miR-30c-5p, and hsa-miR-30b-5p) overlapped from uEVs and renal tissues. In particular, the terms of the distinct upregulated hsa-let-7d-3p target genes were related to ion transport and membrane depolarization, especially in neural precursor cell expressed, developmentally downregulated 4-like (NEDD4L). Real-time PCR of uEVs from another 11 GS patients confirmed significantly elevated hsa-let-7d-3p compared to healthy controls. The decrease in the Nedd4l expression in the collecting duct was also confirmed in NccS707X/S707X knock-in mice. Dual luciferase assays further demonstrated that hsa-let-7d-3p negatively regulated the expression of NEDD4L. These findings concluded that differentially expressed miRNAs could be identified from uEVs in GS patients, and hsa-let-7d-3p, the only upregulated miRNA, negatively regulates NEDD4L expression in the collecting duct.
Keywords: Gitelman syndrome; NEDD4L; extracellular vesicle; hsa‐let‐7d‐3p; microRNA; thiazide‐sensitive sodium chloride cotransporter.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Hsu Y. J., Yang S. S., Chu N. F., Sytwu H. K., Cheng C. J., and Lin S. H., “Heterozygous Mutations of the Sodium Chloride Cotransporter in Chinese Children: Prevalence and Association With Blood Pressure,” Nephrology, Dialysis, Transplantation 24 (2009): 1170–1175. - PubMed
-
- Simon D. B., Nelson‐Williams C., Bia M. J., et al., “Gitelman's Variant of Bartter's Syndrome, Inherited Hypokalaemic Alkalosis, Is Caused by Mutations in the Thiazide‐Sensitive Na‐Cl Cotransporter,” Nature Genetics 12 (1996): 24–30. - PubMed
-
- Bettinelli A., Bianchetti M. G., Girardin E., et al., “Use of Calcium Excretion Values to Distinguish Two Forms of Primary Renal Tubular Hypokalemic Alkalosis: Bartter and Gitelman Syndromes,” Journal of Pediatrics 120 (1992): 38–43. - PubMed
-
- Lin S. H., Shiang J. C., Huang C. C., Yang S. S., Hsu Y. J., and Cheng C. J., “Phenotype and Genotype Analysis in Chinese Patients With Gitelman's Syndrome,” Journal of Clinical Endocrinology and Metabolism 90 (2005): 2500–2507. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

