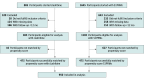
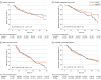
Conflict of Interest Disclosures: Dr Haggiag reported receiving personal fees from Novartis, Merck Serono, Roche, Sanofi, BMS, and Alexion. Dr Prosperini reported receiving personal fees and nonfinancial support from Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, and Viatris. Dr Filippi reported receiving personal fees and/or research support from Alexion, Almirall, Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, the Italian Ministry of Health, the Italian Ministry of University and Research, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and Teva; and participating in advisory boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, and Takeda, as well as scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme. Dr Rocca reported receiving personal fees from AstraZaneca, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Eli Lilly, Genzyme, Horizon Therapeutics Italy, Janssen, Merck Serono SpA, Novartis, Roche, Sanofi, and Teva and research support from the Multiple Sclerosis Society of Canada, the Italian Ministry of Health, the Italian Ministry of University and Research; Dr Rocca is also Associate Editor for Multiple Sclerosis and Related Disorders and Associate Co-Editor for Europe and Africa for Multiple Sclerosis Journal. Dr Iaffaldano reported receiving personal fees from Alexion, Biogen Idec, Bayer, Teva, Roche, BMS, Merck Serono, Novartis and Genzyme. Dr Patti reported receiving personal fees from Alexion, Almirall, Amgen, Biogen, Bristol-Myers and Squibb, Janssen, Merck, Novartis, Roche, Sandoz, Sanofi and grant research support from Biogen, Merck, Novartis, Roche, Sanosi, MIUR, Italian Ministry of Health, and Reload Onlus Association. Dr Inglese reported receiving personal fees from Biogen, Bristol Myers Squibb, Merck, Novartis, Roche, Sanofi, and Janssen. Dr Borriello reported receiving personal fees from Alexion, Biogen, Merck, Sanofi, Novartis, Roche, Teva, and Bristol Myers Squibb. Dr Totaro reported receiving personal fees from and/or serving on advisory boards for Alexion, Almirall, Amgen, Biogen, Lundbeck, Merck Serono, Novartis, Roche, and Sanofi-Genzyme. Dr Lus reported receiving grants and personal fees from Alexion, Amgen, Biogen Idec, Bristol, Merck Serono, Novartis, Sanofi Genzyme, and Roche. Dr Fantozzi reported receiving personal fees from Novartis, Merk-Serono, BMS, Biogen, and Roche. Dr Brescia Morra reported receiving funding and personal fees from Novartis, Roche, Biogen, Teva, Almirall, Sanofi-Genzyme, Merck, Bayer, Mylan, and Bristol Myers Squibb. Dr Romano reported receiving travel support from Biogen and Roche. Dr Frau reported receiving personal fees from Merck Serono, Genzyme, Biogen and Teva and serving on scientific advisory boards for Biogen and Genzyme. Dr Marfia reported receiving fees and grant support from Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, F. Hoffmann–La Roche, Mylan, Alexion, Janssen Cilag, and Bristol Myers Squibb, and serving as an advisory board member for Biogen Idec, Sanofi Genzyme, Merck-Serono, Neurapharm, Novartis, and F. Hoffmann–La Roche; Dr Marfia is also the principal investigator in clinical trials for Biogen Idec, Merck Serono, Novartis, F. Hoffmann–La Roche, Sanofi-Genzyme, Merck Serono, and Bristol Myers Squibb. Dr Maniscalco reported receiving personal fees from Biogen, Merck Serono, Novartis, Roche, Sanofi, Alexion, and Amgen. Dr Amato reported receiving grants from the National Multiple Sclerosis Society, the Canadian Multiple Sclerosis Society, the Italian Health Ministry, Regione Toscana, Bayer, Biogen, Merck, Novartis, Sanofi Genzyme, Teva, Almirall, and Roche, and personal fees for serving as a speaker and member of advisory boards for Bayer, Biogen, Merck, Novartis, Sanofi Genzyme, Teva, Almirall, Roche, Celgene BMS, and Sandoz. Dr Di Sapio reported receiving personal fees from Biogen, Novartis, Roche, Sanofi, Merck Serono, Bristol Meyer Squibb, Janssen, Alexion, Alnylam, and Amgen. Dr De Luca reported receiving personal fees from Merck, Roche, Biogen, and Novartis and serving on the advisory boards for Merck, Roche, and Lundbeck. Dr Grisafulli reported receiving support for congress participation from Mylan, Merck-Serono, Novartis, and Roche, and serving on the advisory boards for Novartis and Roche. Dr Foschi reported receiving personal fees from Roche, Novartis, Biogen, Sanofi, Merck, and Bristol-Myers, and serving as a consultant for Roche and Novartis. Dr Cavalla reported receiving person fees from Biogen, Merck-Serono, Novartis, Roche, Sanofi/Genzyme, and Teva and serving as an advisory board member for Almirall, Biogen, Merck-Serono, Sanofi-Genzyme, Roche, and Teva. Dr Conte reported receiving personal fees from Roche, Biogen, Novartis, BMS, Allmiral, Merck, and Lundbeck and research support from Biogen. Dr Valentino reported receiving personal fees from Roche, Merck, and Novartis. Dr Ferraro reported receiving personal fees from Alexion. Dr Lugaresi reported receiving personal fees from Alexion, Amgen/Horizon, Biogen, Bristol-Myers Squibb/Celgene, Janssen/Johnson&Johnson, Merck Serono, Novartis, Roche, and Sanofi/Genzyme. Dr Perini reported receiving personal fees from Merck, Biogen, Sanofi-Aventis, Novartis-Pharma, Teva, Roche, Almirall, and Alexion, and serving as a consultant and member of advisory boards for Biogen, Genzyme, Merck, Almirall, Merck, Roche, and Novartis. Dr Ferraro reported receiving personal fees from Bristol-Myers Squibb, Merck Serono, Novartis, Roche, and Sanofi. Dr Avolio reported receiving personal fees and grants from Roche, Novartis, Sanofi, Bristol-Myers Squibb, Biogen, Merck, Alexion, and Amgen, and serving as an advisory board member of Roche, Novartis, Sanofi, Bristol-Myers Squibb, Biogen, Merck, Alexion, and Amgen. Dr Vianello reported receiving personal fees from Biogen, Genzyme, Bristol-Myers Squibb, Serono, Merck, Novartis, Roche, and Janssen. Dr Marinelli reported receiving personal fees from Merck Serono, Bristol-Myers Squibb, Novartis, and Roche. Dr Pasquali reported receiving fees and grants from Biogen, Sanofi-Genzyme, Novartis, Alexion, Roche, and Merck Serono, and participating in advisory boards from Merck and Sanofi-Genzyme, Dr Bucello reported receiving personal fees from Roche and Novartis; serving as a consultant for Biogen, Sanofi, Merck Serono, and Novartis; and participating in speakers’ bureaus for Biogen, Bristol-Myers Squibb, Novartis, Merck, Sanofi, and Roche. Dr Vecchio reported receiving fees from Novartis, Sanofi, Roche and Merck. and research support from Fondazione Italiana Sclerosi Multipla paid to their institution. Dr Sangalli reported receiving fees from Alexion, Biogen, Bristol-Myers Squibb, Janssen, Merck Serono, Novartis, Roche, and Sanofi. Dr Rovaris reported receiving fees from Merck, Bristol-Myers Squibb, and Roche. Dr De Riz reported receiving personal fees from Merck-Serono, Novartis, Roche, Sanofi, Biogen, Teva, Almirall, Bristol-Myers Squibb, and Horizon Therapeutics. Dr Grimaldi reported receiving personal fees from Merck, Biogen Idec, Sanofi-Aventis, Teva, Roche, Novartis, Alexion and Bayer. Dr Ardito reported receiving personal fees from Bristol-Myers Squibb, Novartis, Sanofi Aventis, Alexion, Genzyme, and Lusofarmaco, and serving as a consultant for Biogen and Novartis. Dr Sinisi reported receiving personal fees from Merck Serono, Bristol-Myers Squibb, and Johnson&Johnson, and participating in advisory boards with Novartis and Merck Serono. Dr Immovilli reported receiving personal fees from Roche, Novartis, Bristol-Myers Squibb, Jansen, Sanofi, Merck, and Alexion. Dr Pesci reported receiving fees from Merck, Biogen, Roche, Novartis, and Bristol. Dr Colombo reported receiving personal fees from Sanofi, Biogen, Novartis, Merck, Roche, Bristol-Myers Squibb, Janssen, and Alexion. Dr Coniglio reported receiving personal fees from Biogen, Bristol-Myers Squibb, Merck Serono, Novartis, Roche, and Sanofi Genzyme. Dr Cargnelutti reported receiving personal fees from Roche, Novartis, Merck, Sanofi Genzyme, and Biogen. Dr Matta reported receiving honoraria from Novartis, Merck, Sanofi, Genzyme, and Roche. Dr Falcini reported receiving travel grants from Novartis, Biogen, Roche, and Alexion. Dr Mascoli reported receiving personal fees from Novartis and Biogen. Dr Solaro reported receiving personal fees from Merck, Biogen, Novartis, Roche, and Almirall. Dr Sica reported serving as an advisory board member of Novartis, Biogen, Bristol-Myers Squibb, and Merck Serono. Dr Cerqua reported receiving personal fees from Roche, Novartis, Sanofi Genzyme, Alexion, Jansenn, and Merck Serono. Dr Rini reported receiving personal fees from Novartis, BIAL, Abbvie, and Sanofi. Dr Zuliani reported receiving personal fees from Alexion, Almirall, Biogen, Genzyme, Merck-Serono, Mylan, Novartis, and Roche. Dr Ruggieri reported receiving personal fees from Biogen, Bristol-Myers Squibb, Merck, Novartis, Alexion and Roche. Dr Gasperini reported receiving personal fees from Biogen, Merck-Serono, Teva, Sanofi, Novartis, Genzyme, and Alexion. Dr Trojano reported receiving personal fees from Biogen, Novartis, Roche, Merck, Sanofi, and Bristol-Myers Squibb. Dr Tortorella reported receiving honoraria from Biogen, Bristol-Myers Squibb, Merck, Novartis, Alexion, Amgen and Roche for consulting services, speaking and/or travel support. No other disclosures were reported.