Lung endothelial PEAR1 induces tumor cell dormancy
- PMID: 41185039
- PMCID: PMC12581312
- DOI: 10.1186/s12943-025-02488-3
Lung endothelial PEAR1 induces tumor cell dormancy
Abstract
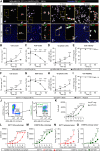
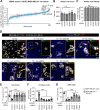
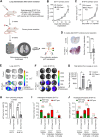
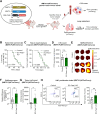
In many cancer patients, distant metastases develop after years of dormancy. Understanding how disseminated tumor cells (DTCs), which are often found in proximity to the microvasculature, remain dormant and what regulates their reactivation is one of the major challenges in tumor biology. In a screen for endothelial secreted and plasma membrane proteins able to regulate tumor cell dormancy, we identified the transmembrane protein platelet and endothelial aggregation receptor 1 (PEAR1). Human and murine endothelial cells lacking PEAR1 lost the ability to promote dormancy of different tumor cells, and the extracellular part of PEAR1 was able to rescue this effect. Similarly, in mice lacking PEAR1 in endothelial cells, tumor cell dormancy in the lung was reduced and tumor metastasis was increased. We found that PEAR1 induces tumor cell dormancy by binding lysyl oxidase like 2 (LOXL2) and cathepsin D (CTSD), which both inhibit tumor cell dormancy and promote tumor growth and metastasis. Tumor cells with suppressed CTSD expression showed increased dormancy and decreased metastatic potential in vivo. Our data identify a mechanism underlying tumor cell dormancy and suggest CTSD and LOXL2 as targets for approaches to promote dormancy.
Keywords: CTSD; Dormancy; Endothelial; LOXL2; Metastasis; PEAR1; p27.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures involving animal care and use in this study were approved by the local animal ethics committee (Regierungspräsidium Darmstadt, Germany) (B2-2051; B2-1237; B2-2003; B2-1175; B2-1178). Consent for publication: All authors have consented to submit this article for publication. Conflict of interest: The authors declare no conflicts of interest related to this work. Competing interests: The authors declare no competing interests.
Figures



References
-
- Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer. 2020;20:681–94. 10.1038/s41568-020-00300-6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

