Protan triggered colorimetric and fluorometric responsive coumarin coupled imidazole as Co2+ sensor, DFT and zebrafish bioimaging studies
- PMID: 41188313
- PMCID: PMC12586424
- DOI: 10.1038/s41598-025-22551-9
Protan triggered colorimetric and fluorometric responsive coumarin coupled imidazole as Co2+ sensor, DFT and zebrafish bioimaging studies
Abstract
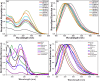
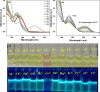
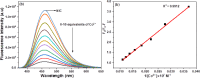
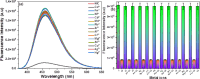
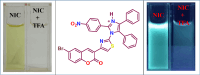
This study elucidates a novel Co2+ ion fluorescence chemosensor 6-bromo-3-(2-(2-(4-nitrophenyl)-4,5-diphenyl-1H-imidazol-1-yl)thiazol-4-yl)-2H-chromen-2-one (NIC), which integrates imidazole and coumarin-thiazole moiety evolved over other frequently occurring metal ions. Fluorescence spectra are specifically used to study the complexation of NIC with different metal ions in an acetonotrile: water mixture (8:2) at room temperature. When Co2+ was present, NIC shows a notable quenching of fluorescence at 450 nm as a result of a complex forming which was accompanied by the emergence of a new band at 515 nm in the uv visible spectrum. Also, the colour of NIC was changed from yellow to brown which was evident from naked eyes. The sensor NIC demonstrated its high sensitivity towards Co2+ ions with a limit of detection (LOD) of 7 µM. The Job's plot validates the 1:1 binding stoichiometry between Co2+ and NIC. The chemosensor NIC also exhibited colorimetric and fluorometric sensing ability with a visible colorimetric response to Trifluoroacetic acid (TFA). Further, the study was also validated with zebrafish bioimaging studies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

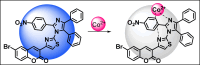





References
-
- Wakabayashi, K., Yorimitsu, H. & Oshima, K. Cobalt-catalyzed tandem radical cyclization and cross-coupling reaction: its application to benzyl-substituted heterocycles. J. Am. Chem. Soc.123, 5374–5375 (2001). - PubMed
-
- Okamoto, S. & Eltis, L. D. The biological occurrence and trafficking of Cobalt. Metallomics3, 963–970 (2011). - PubMed
-
- Kim, B. E., Nevitt, T. & Thiele, D. J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol.4, 176–185 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

