Use of rescue noninvasive ventilation for post-extubation respiratory failure
- PMID: 41188988
- PMCID: PMC12584535
- DOI: 10.1186/s13054-025-05689-w
Use of rescue noninvasive ventilation for post-extubation respiratory failure
Abstract
Background: Robust evidence supports the use of preemptive non-invasive ventilation (NIV) after extubation in selected high-risk patient cohorts. In contrast, current guidelines discourage the use of NIV as a rescue therapy for respiratory failure that develops later after extubation, based on earlier studies indicating a potential increase in hospital mortality due to delayed reintubation. Nonetheless, NIV continues to be employed in this setting. We conducted a post-hoc analysis of a randomized trial to assess the clinical outcomes of rescue NIV for post-extubation respiratory failure.
Methods: In this post-hoc analysis of a randomized trial comparing high-flow with Venturi mask oxygen in hypoxemic patients after extubation, we included those who developed post-extubation respiratory failure according to prespecified criteria; patients who received rescue NIV per physician's decision were compared to those who received direct re-intubation. Criteria for re-intubation during NIV were prespecified. Odds ratio after inverse probability of treatment weighting and posterior probabilities by Bayesian regression are reported.
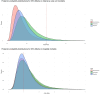
Results: Among 494 extubated patients, 147 developed respiratory failure while receiving oxygen therapy, occurring at a median of 37 h [IQR 13-85] after extubation: 83 (57%) were treated with rescue NIV and 64 (43%) received immediate re-intubation. The rate of NIV failure was 58%, without differences between patients with hypoxemic respiratory failure and those with hypercapnia and/or respiratory distress (60% vs. 56%, p = 0.82). In the weighted cohort, the use of rescue NIV, compared to direct re-intubation, was associated with lower intensive care unit mortality (adjusted odds ratio = 0.31 [95%CI: 0.12-0.82], p = 0.019) and similar hospital mortality (adjusted odds ratio = 1.01 [95%CI: 0.43-2.33], p = 0.99). The posterior probability that NIV reduced intensive care unit mortality was > 90% across all priors. The posterior probability that NIV did not increase hospital mortality was 44% under a noninformative prior, 47% under a skeptical prior, and 39% under a pessimistic prior.
Conclusion: Rescue NIV for post-extubation respiratory failure is associated with high failure rates; however, when applied with well-defined criteria for reintubation, it does not appear to be clearly associated with increases in hospital mortality. A randomized trial to re-evaluate the efficacy of rescue NIV for post-extubation respiratory failure is warranted.
Clinical trial registration: Registered at clinicaltrials.gov (NCT02107183) on April 8th, 2014.
Keywords: Noninvasive ventilation; Post-extubation respiratory failure; Weaning.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate : The institutional review board of the coordinator center (Catholic University of The Sacred Heart, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy) reviewed and approved the study protocol before trial initiation (n. 12634/13 on December 5th, 2013). By in-site beginning of enrolment, each local ethics committee reviewed and approved the study protocol. All enrolled patients provided written informed consent in accordance with committee recommendations. Consent for publication: Not applicable. Competing interests: DLG has received payments for travel expenses by Getinge, Draeger and Hamilton, research grants by Fisher and Paykel and GE, and non-financial support for research by Intersurgical, Dimar and Harol. SJ reports receiving consulting fees from Draeger, Medtronic, Baxter, Fresenius-Xenios, and Fisher & Paykel. A Demoule reports grants from the French Ministry of Health, Lungpacer, Respinor, Liberate Medical, consulting fees from Respinor, Liberate Medical, SAT Lutech, payment or honoraria for lectures, presentations from Fisher & Paykel, Astra, support for attending meetings and/or travel from Respinor, outside the submitted work JDR reports consulting fees, covering of travel expenses and equipment support by Fisher & Paykel. PN research laboratory has received equipment and grants from Draeger, Gilead and Intersurgical S.p.A. He also received honoraria/speaking fees from Gilead, GSK, Shionogi, Getinge, Mindray. He contributed to the development of the helmet Next, whose license for patent belongs to Intersurgical S.P.A. and receives royalties for that invention. SH reports speaking fees by MSD, Shionogi, Advanz Pharma Biomerieux. JPF reports having received grants from the French Ministry of Health, outside the submitted work; grants, personal fees and non-financial support from Fisher & Paykel HealthCare, outside the submitted work; personal fees and non-financial support from SOS Oxygène, outside the submitted work. VL belongs to a research group which received fees from Gilead, Pfizer, Alexion, Sanofi, MSD and personal fees and covering for travel expenses by Biomerieux. LB’S laboratory has received support for research by Covidien (PAV), Air Liquide (CPR), Philips (equipment for sleep), Fisher & Paykel (high flow therapy) and GE healthcare. MA has received payments for Board participation from Menarini, and Shionogi, and a research grant by General Electric Healthcare. And Fisher & Paykel SMM discloses having received speaking fees by GE Healthcare, Masimo, Getinge and Aspen, fees for advisory board participation by Sanofi, and a research grant, support for travel expenses and receipt of materials by Fisher and Paykel.
Figures




References
-
- Ferreyro BL, De Jong A, Grieco DL. How to use facemask noninvasive ventilation. Intensive Care Med. 2024;50:1346–9. - PubMed
-
- Fernando SM, Tran A, Sadeghirad B, Burns KEA, Fan E, Brodie D, et al. Noninvasive respiratory support following extubation in critically ill adults: a systematic review and network meta-analysis. Intensive Care Med. 2022;48:137–47. - PubMed
-
- Ferrer M, Esquinas A, Arancibia F, Bauer TT, Gonzalez G, Carrillo A, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med. 2003;168:70–6. - PubMed
-
- Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of Postextubation High-Flow Nasal Cannula vs Noninvasive Ventilation on Reintubation and Postextubation Respiratory Failure in High-Risk Patients: A Randomized Clinical Trial. JAMA [Internet]. 2016 316 1565 74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26975498 - PubMed
-
- Thille AW, Coudroy R, Nay M-A, Gacouin A, Decavèle M, Sonneville R, et al. Beneficial Effects of Noninvasive Ventilation after Extubation in Obese or Overweight Patients: A Post Hoc Analysis of a Randomized Clinical Trial. Am J Respir Crit Care Med [Internet]. 2022 205 440 9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34813391 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

