A novel methodological approach to understanding the cortical and subcortical effects of aerobic exercise in Parkinson's disease
- PMID: 41189627
- PMCID: PMC12580177
- DOI: 10.3389/fnhum.2025.1657049
A novel methodological approach to understanding the cortical and subcortical effects of aerobic exercise in Parkinson's disease
Abstract
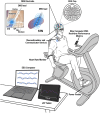
Introduction: Aerobic exercise mitigates symptoms of Parkinson's disease (PD) and may slow disease progression; however, the neural mechanisms underlying these improvements are not well understood. In this study, we discuss the methodology for simultaneously recording local field potentials (LFP) from the subthalamic nucleus (STN), cortical activity using scalp electroencephalography (EEG), and exercise performance metrics during a 40-min aerobic cycling session. Data from a single patient with PD are presented to illustrate the utility, feasibility, and data integrity of the experimental set up.
Methods: The Medtronic Percept™ DBS system was used to record and stream bilateral STN-LFP in the OFF-therapy condition (OFF-DBS and OFF-antiparkinson medications) during a 40-min aerobic exercise session. A 64-channel mobile EEG system recorded cortical data. The neural data streams were synchronized using a TENS device that injected a specified electrical signal into the EEG and LFP recordings. Exercise performance metrics, heart rate, cadence, and power were synchronized with neural data and collected during the exercise session. The study is registered on ClinicalTrials.gov, trial identifying numbers NCT05905302 and NCT05972759.
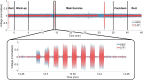
Results: STN-LFP, EEG, and exercise performance data can be synchronized, recorded for more than 40 min, and analyzed to evaluate how aerobic exercise impacts patterns of cortical and subcortical neural activity.
Conclusion: While exercise positively affects symptoms of PD, the precise effects of exercise on network activity remain unclear. The methods utilized for collecting and analyzing neural (cortical and subcortical) and exercise-related data during a typical bout of aerobic exercise suggest that this approach can be adopted for larger, long-term exercise studies in patients with PD and deep brain stimulation (DBS). The described protocol provides a roadmap for future projects aiming to combine STN-LFP and cortical data to better understand how exercise may alter cortico-basal-ganglia-thalamic dynamics in PD.
Keywords: Parkinson’s disease; aerobic exercise; electroencephalogram; local field potential; subthalamic nucleus.
Copyright © 2025 Koop, Rosenfeldt, Berki, Bazyk, Davidson, Malan, Nagel, Walter, Liao and Alberts.
Conflict of interest statement
JA has authored intellectual property associated with forced exercise technology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bronte-Stewart H., Barberini C., Koop M. M., Hill B. C., Henderson J. M., Wingeier B. (2009). The STN beta-band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp. Neurol. 215, 20–28. doi: 10.1016/j.expneurol.2008.09.008, PMID: - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

