Liquid biopsy in gastrointestinal oncology: clinical applications and translational integration of ctDNA, CTCs, and sEVs
- PMID: 41190015
- PMCID: PMC12580207
- DOI: 10.3389/or.2025.1702932
Liquid biopsy in gastrointestinal oncology: clinical applications and translational integration of ctDNA, CTCs, and sEVs
Abstract
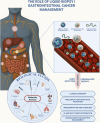
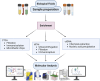
Background and aims: Liquid biopsy offers a minimally invasive tool to detect actionable mutations, monitor minimal residual disease (MRD), and guide therapy in gastrointestinal (GI) cancers. We critically review the clinical utility of circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and small extracellular vesicles (sEVs) across GI malignancies and propose a framework for their integration into clinical practice.
Methods: We synthesized evidence from over 200 studies, including prospective trials and translational research, to assess diagnostic accuracy, prognostic value, and clinical actionability of each biomarker type in esophageal, gastric, colorectal, pancreatic, hepatocellular, and biliary cancers.
Results: ctDNA has shown strong potential for MRD detection and treatment monitoring, particularly in colorectal and pancreatic cancer. CTCs offer insights into metastatic risk and therapeutic resistance, while sEVs provide molecular cargo relevant to immunomodulation and disease progression. Emerging microfluidics and AI-driven multi-omics approaches may overcome current limitations.
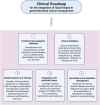
Conclusion: The integration of liquid biopsy technologies into GI oncology holds promise for early detection and precision therapy. We propose a five-phase clinical roadmap and outine the key research gaps that need to be addressed before widespread implementation in routine care.
Keywords: circulating tumor DNA (ctDNA); circulating tumor cells (CTC); extracellular vesicles (EVs); gastrointestinal cancer (GI); liquid biopsy.
Copyright © 2025 Palieri, De Luca, Balestra, Panzetta, Lotesoriere, Rizzi, Ricci, Mastrogiacomo, Curri, Laghi, Giannelli, Depalo and Scavo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

