Transforming microfluidics for single-cell analysis with robotics and artificial intelligence
- PMID: 41190506
- PMCID: PMC12587405
- DOI: 10.1039/d5lc00216h
Transforming microfluidics for single-cell analysis with robotics and artificial intelligence
Abstract
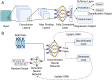
Single-cell analysis has advanced biomedical research by revealing cellular heterogeneity with unprecedented resolution, identifying rare subpopulations that drive disease progression and therapeutic resistance. Microfluidics is central to this advancement, enabling precise single-cell isolation, manipulation, and cellular profiling. However, limitations in automation, reliability, and technical barriers hinder the widespread adoption of microfluidic single-cell analysis. This review highlights key innovations in experimental methods and deep learning-driven data analysis to overcome these challenges. Operating microfluidics with robotic operation, digital microfluidics, or microrobots enhances experimental precision and scalability. Beyond experimental automation, deep learning revolutionizes data interpretation through label-free image processing and cell status classification and regression. Generative models further refine analysis by correcting batch effects and generating synthetic datasets, improving accuracy and reproducibility in single-cell studies. Considering the complexity of integrating these technologies, remote shared cloud labs represent a potential pathway toward standardized and high-throughput single-cell analysis, facilitating broader access to advanced experimental workflows. Overall, the convergence of robotics and artificial intelligence in single-cell analysis will change data acquisition, hypothesis testing, and model refinement, driving breakthroughs in drug discovery and personalized medicine. While implementation remains challenging, this paradigm shift is transforming biomedical research, enabling unprecedented precision, scalability, and data-driven innovation.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








References
-
- Cho S. K. Moon H. J. Kim C. J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003;12(1):70–80. doi: 10.1109/JMEMS.2002.807467. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

