Rapid improvements in glycemic management with use of continuous glucose monitoring in adults with type 2 diabetes treated with basal insulin: 3-month analysis of the MOBILE study
- PMID: 41192933
- PMCID: PMC12587997
- DOI: 10.1136/bmjdrc-2025-005469
Rapid improvements in glycemic management with use of continuous glucose monitoring in adults with type 2 diabetes treated with basal insulin: 3-month analysis of the MOBILE study
Abstract
Introduction: This analysis investigated whether use of real-time continuous glucose monitoring (CGM) compared with blood glucose monitoring (BGM) results in rapidly improved glycemic management in adults with type 2 diabetes (T2D) treated with basal insulin.
Research design and methods: Using data from the MOBILE study where adults (n=175) with T2D treated with basal insulin without prandial insulin were randomized (2:1) to either CGM (n=116) or BGM (n=59), the treatment effect on glycemic management was determined over 3 months. The main outcome was a between-group difference in hemoglobin A1c (HbA1c) at 3 months adjusted for baseline value. Other outcomes included changes in CGM-derived glucose metrics and hypoglycemic events.
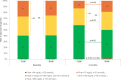
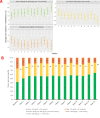
Results: After 3 months, there was a greater reduction from baseline in mean HbA1c in the CGM group compared with the BGM group, from 9.1±1.0% (76±11 mmol/mol) to 8.0±1.2% (64±13 mmol/mol) in the CGM group and from 9.0±0.9% (75±10 mmol/mol) to 8.5±1.5% (69±16 mmol/mol) in the BGM group (adjusted difference, -0.6% (95% CI -0.9% to -0.3%); -6.6 mmol/mol (95% CI -10.2 to -2.9), p<0.001). Mean time spent in range 70-180 mg/dL (3.9-10.0 mmol/L) increased significantly more in the CGM group than the BGM group (adjusted difference, +9.3% (95% CI 2.1% to 16.4%), p<0.001). There also was a greater reduction in mean time >250 mg/dL (>13.9 mmol/L) with CGM (adjusted difference, -5.8% (95% CI -10.4% to -1.2%), p<0.001) without an increase in time <70 mg/dL (<3.9 mmol/L). Mean weekly hypoglycemic event rate was lower with CGM than BGM (adjusted difference, -0.2 events per week (95% CI -0.4 to -0.1), p<0.001). Further, in the CGM group, significant improvements in CGM metrics were observed during the first 7 days of CGM use.
Conclusions: In adults with basal insulin-treated T2D, use of CGM compared with BGM resulted in rapidly improved glycemic management, with a substantial reduction in HbA1c over 3 months.
Trial registration number: NCT03566693.
Keywords: Blood Glucose Self-Monitoring; Diabetes Mellitus, Type 2; Glycated Hemoglobin A.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: TWM has received research support, acted as a consultant, or served on a scientific advisory board for Abbott Diabetes Care, Dexcom, Medtronic, Novo Nordisk, Sanofi, Lilly, Insulet, Sequel, Amgen, Luna Diabetes, and Zealand. TWM’s employer, HealthPartners Institute, contracts for his services and no personal income from these services goes to TWM. RWB reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding, study supplies, and consulting fees from Insulet, Tandem Diabetes Care, and Beta Bionics; grant funding and study supplies from Dexcom and Abbott; grant funding from Bigfoot Biomedical, embecta, Sequel Med Tech, and MannKind; study supplies from Medtronic; consulting fees and study supplies from Novo Nordisk; consulting fees from Vertex, Hagar, DreaMed, Ypsomed, Abata Therapeutics, Eli Lilly and Zucara. RMB has received research support, acted as a consultant, or served on a scientific advisory board for Abbott Diabetes Care, Ascensia, CeQur, Dexcom, Eli Lilly, Insulet, Luna Health, Medtronic, Novo Nordisk, Roche-Genentech, Sanofi, and Tandem Diabetes Care, RMB’s employer, HealthPartners Institute, contracts for his services and no personal income from these services goes to RMB. CG, JD, KE-H, MLJ, JRC, and SEB are employees of Dexcom, Inc.
Figures
References
-
- World Health organization Global report on diabetes. 2016
-
- NHS Digital National diabetes audit core report 1: care processes and treatment targets 2022-23, underlying data. 2023
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
