Phospholipid scramblases TMEM16F and Xkr8 mediate distinct features of phosphatidylserine (PS) externalization and immune suppression to promote tumor growth
- PMID: 41198619
- PMCID: PMC12592367
- DOI: 10.1038/s41420-025-02789-y
Phospholipid scramblases TMEM16F and Xkr8 mediate distinct features of phosphatidylserine (PS) externalization and immune suppression to promote tumor growth
Abstract
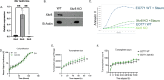
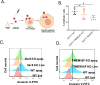
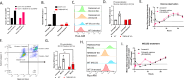
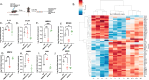
The phospholipid scramblases Xkr8 and TMEM16F externalize phosphatidylserine (PS) by distinct mechanisms. Xkr8 is activated by caspase-mediated proteolytic cleavage and, in synergy with the inactivation of P4-ATPase flippases, results in the irreversible externalization of PS on apoptotic cells and an "eat-me" signal for efferocytosis. In contrast, TMEM16F is a calcium-activated scramblase that reversibly externalizes PS on viable cells via the transient increase in intracellular calcium in live cells. The tumor microenvironment (TME) is abundant with exposed PS, resulting from prolonged oncogenic and metabolic stresses and high apoptotic indexes of tumors. Such chronic PS externalization in the TME has been linked to host immune evasion from interactions of PS with inhibitory PS receptors, such as TAM and TIM family receptors. Here, in an effort to better understand the contributions of apoptotic vs live cell PS-externalization to tumorigenesis and immune evasion, we employed an EO771 orthotopic breast cancer model and genetically ablated Xkr8 and TMEM16F using CRISPR/Cas9. While neither the knockout of Xkr8 nor TMEM16F showed defects in cell intrinsic properties related to proliferation, tumor-sphere formation, and growth factor signaling, both knockouts suppressed tumorigenicity in immune-competent mice, but not in NOD/SCID or RAG-knockout immune-deficient strains. Mechanistically, Xkr8-KO tumors suppressed macrophage-mediated efferocytosis, and TMEM16F-KO suppressed ER stress/calcium-induced PS externalization. Our data support an emerging idea in immune-oncology that constitutive PS externalization, mediated by scramblase dysregulation on tumor cells, supports immune evasion in the tumor microenvironment. This links apoptosis/efferocytosis and oncogenic stress involving calcium dysregulation, contributing to PS-mediated immune escape and cancer progression.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: SVK and RBB are cofounders of a biotechnology company called Targeron Therapeutics, LLC, that aims to develop PS-targeting IFNs for immune-oncology applications.
Figures




Update of
-
Phospholipid Scramblases TMEM16F and Xkr8 mediate distinct features of Phosphatidylserine (PS) externalization and immune suppression to promote tumor growth.bioRxiv [Preprint]. 2025 Jun 23:2025.04.17.649445. doi: 10.1101/2025.04.17.649445. bioRxiv. 2025. Update in: Cell Death Discov. 2025 Nov 6;11(1):506. doi: 10.1038/s41420-025-02789-y. PMID: 40391322 Free PMC article. Updated. Preprint.
References
-
- Bergelson LD, Barsukov LI. Topological asymmetry of phospholipids in membranes. Science. 1977;197:224–30. - PubMed
-
- Rothman JE, Lenard J. Membrane asymmetry. Science. 1977;195:743–53. - PubMed
-
- Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–27. - PubMed
-
- Finkielstein CV, Overduin M, Capelluto DG. Cell migration and signaling specificity is determined by the phosphatidylserine recognition motif of Rac1. J Biol Chem. 2006;281:27317–26. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

