Unraveling the mechanisms behind the enhanced efficacy of β-lactam-based sideromycins
- PMID: 41198856
- PMCID: PMC12592502
- DOI: 10.1038/s42003-025-08898-9
Unraveling the mechanisms behind the enhanced efficacy of β-lactam-based sideromycins
Abstract
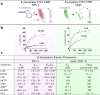
Previous studies have explored combining β-lactams with siderophores to create Trojan horse molecules that can penetrate the outer membrane of Gram-negative bacteria via TonB-dependent transporter (TBDT). While the main advantage explaining their enhanced antibiotic activity is believed to be improved membrane permeability, other factors remain underexplored. This study evaluates three siderophore-β-lactam compounds: a bis-catechol siderophore linked to ampicillin or loracarbef, and a mixed bis-catechol-mono-hydroxamate siderophore linked to cefaclor. Minimal inhibitory concentrations showed that siderophore conjugation could enhance β-lactam efficacy by over 8000-fold. Comparison with unconjugated β-lactams revealed a complex interplay between β-lactamase susceptibility, competition with endogenous siderophore, membrane uptake, and binding to penicillin-binding proteins (PBPs). Enhanced PBP binding, particularly in Escherichia coli, emerged as a key factor contributing to improved bacterial inhibition by siderophore-β-lactam conjugates. Overall, the study provides insights into how siderophore conjugation enhances β-lactam activity and the therapeutic potential of the conjugates as narrow or broad-spectrum antibiotics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. L.R., Y.-M.L., M.G., and M.J.M. are associated with Hsiri Therapeutics, which provided the synthetic siderophores used in this study. No funds were obtained from Hsiri.
Figures

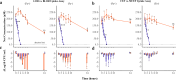
 ) or bis-catechol-loracarbef (BLOR) (
) or bis-catechol-loracarbef (BLOR) ( ) and of b cefaclor (CEF) (
) and of b cefaclor (CEF) ( ) or mixed bis-catechol-mono-hydroxamate-cefaclor (MCEF) (
) or mixed bis-catechol-mono-hydroxamate-cefaclor (MCEF) ( ), in MHBCA (Fe+) and iron-deprived (ID)-MHBCA (Fe-), over a 24-h period. Net concentrations represent the levels of each antibiotic measured in the presence of E. coli, corrected by subtracting the corresponding concentrations measured in the absence of bacteria at each time point. Normality was verified with the Shapiro–Wilk’s test and the statistical analysis is a comparison of repeated measures ANOVA between each time point relatively to the 0-h time point (n = 3). BLOR showed a statistically significant uptake at 24 h (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001), but MCEF uptake was not significant (ns). The Δlog10 CFU/mL (bars with individual data points) was calculated by subtracting the bacterial count with drugs to the bacterial count without drugs (normal growth) at each time point. Panel c is the Δlog10 CFU/mL of LOR (blue) and BLOR (orange), and d is the Δlog10 CFU/mL of CEF (blue) and MCEF (orange).
), in MHBCA (Fe+) and iron-deprived (ID)-MHBCA (Fe-), over a 24-h period. Net concentrations represent the levels of each antibiotic measured in the presence of E. coli, corrected by subtracting the corresponding concentrations measured in the absence of bacteria at each time point. Normality was verified with the Shapiro–Wilk’s test and the statistical analysis is a comparison of repeated measures ANOVA between each time point relatively to the 0-h time point (n = 3). BLOR showed a statistically significant uptake at 24 h (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001), but MCEF uptake was not significant (ns). The Δlog10 CFU/mL (bars with individual data points) was calculated by subtracting the bacterial count with drugs to the bacterial count without drugs (normal growth) at each time point. Panel c is the Δlog10 CFU/mL of LOR (blue) and BLOR (orange), and d is the Δlog10 CFU/mL of CEF (blue) and MCEF (orange).

References
-
- Neu, H. C. β-lactam antibiotics: structural relationships affecting in vitro activity and pharmacologic properties. Rev. Infect. Dis.8, S237–S259 (1986). - PubMed
-
- Web Annex. Infographics. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book (World Health Organization, 2022).
-
- Ito, A. et al. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J. Antimicrob. Chemother.71, 670–677 (2016). - PubMed
-
- Hider, R. C. & Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep.27, 637–657 (2010). - PubMed
MeSH terms
Substances
Grants and funding
- 2019-PR-255463/Fonds de Recherche du Québec - Nature et Technologies (Quebec Fund for Research in Nature and Technology)
- https://doi.org/10.69777/334786/Fonds de Recherche du Québec - Nature et Technologies (Quebec Fund for Research in Nature and Technology)
- 2020-04811/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- BESD3-556674-2020/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

