Prevalence of Hepatitis E genotype 3 among liver disease patients in Southwestern Nigeria
- PMID: 41199314
- PMCID: PMC12590905
- DOI: 10.1186/s12985-025-02989-z
Prevalence of Hepatitis E genotype 3 among liver disease patients in Southwestern Nigeria
Abstract
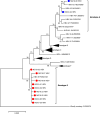
Owing to its high mortality rate, viral hepatitis is a major public health problem, especially in low-income countries. In Africa, hepatitis B virus (HBV) and hepatitis E virus (HEV) are highly endemic, and HBV/HEV coinfections, which are associated with more severe liver disease and poor outcomes, are common. HEV genotypes 1 and 2 have been associated with large human outbreaks, while 3 is known to circulate in pigs and sporadically in humans. In this study, the prevalence of HBV and HEV among individuals with acute or chronic liver diseases in Osun State, Southwest Nigeria, was analyzed. One hundred plasma samples from liver disease patients attending Ladoke Akintola University Teaching Hospital were analyzed for the presence of anti-HEV antibodies and hepatitis B surface antigen (HBsAg) via ELISA, and HEV RNA and HBV DNA were analyzed via RT‒PCR. Virus genotyping was performed by sequencing and subsequent phylogenetic analysis. Overall, 50 individuals (50%) were positive for HBsAg, of which 14 (28%) also tested positive for HBV DNA. Two individuals (2%) had occult HBV infection. Most HBV strains were genotype E, except for two genotype A (A2 and A3). Anti-HEV antibodies were detected in eight individuals (8%), with one (1%) being positive for anti-HEV IgM and seven (7%) for anti-HEV IgG. Nine (9%) samples had detectable HEV RNA, with one being HEV-3; a rare occurrence in Nigeria. Coinfection with HBV/HEV was detected in seven (7%) individuals. The prevalence of HEV in Nigeria is low, but considering the high prevalence of HBV and the possible complications due to HEV coinfection or superinfection, HEV screening and HBV vaccination targeting high-risk populations are emphasized.
Keywords: Coinfection; HBV; HEV; Liver disease; Nigeria.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed written consent was obtained from all participants, and the study was approved by the Osun State Health Research Ethics Committee (OSHREC/PRS/569T/52). Consent for publication: Informed consent was obtained from the participants for the publication of the data included in this article. Competing interests: The authors declare no competing interests.
Figures


References
-
- Kilonzo SB, Gunda D, Ning Q, Han M. Where hepatitis B and hepatitis E meet: epidemiological and clinical aspects. Hepat Monthly. 2019;19(10):e93840. 10.5812/hepatmon.93840
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

