Prognostic evaluation of glycolysis markers in hepatocellular carcinoma: insights from meta-analysis and multi-omics approaches
- PMID: 41206438
- PMCID: PMC12595624
- DOI: 10.1186/s12920-025-02253-x
Prognostic evaluation of glycolysis markers in hepatocellular carcinoma: insights from meta-analysis and multi-omics approaches
Abstract
Background: Glycolysis, a central process of cellular energy metabolism, has been shown to be closely associated with the development of hepatocellular carcinoma (HCC). This study aimed to investigate the prognostic value of the glycolysis gene set (GGS) in HCC.
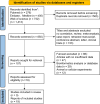
Methods: Online databases were searched to identify studies on the correlation between glycolysis-related gene signature score and clinical characteristics in patients with HCC. HR and OR values with 95% CI were calculated. Bioinformatics analysis and in vitro validation were used to validate the results of the meta-analysis and investigate the potential oncogenic mechanisms of GGS.
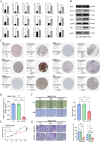
Results: Nineteen studies involving 3,406 patients were included. The pooled analysis showed that a high glycolysis-related gene signature score was associated with poor overall survival (OS) (HR = 1.98, 95% CI 1.59-2.46, P < 0.001), disease-free survival (DFS) (HR = 2.02, 95% CI 1.54-2.64, P < 0.001), and relapse-free survival (RFS) (HR = 2.38, 95% CI 1.39-4.08, P = 0.002). Bioinformatic and in vitro experiments confirmed the prognostic relevance and differential expression of GGS in HCC, and functional assays of ENO1 further demonstrated its role in HCC progression.
Conclusion: The upregulation of the glycolysis-related gene signature score is predominantly associated with poor prognosis in patients with HCC, suggesting that GGS may serve as a potential prognostic biomarker and therapeutic target for HCC, as exemplified by ENO1 functional validation.
Keywords: Biomarker; Glycolysis gene set; Hepatocellular carcinoma; Prognosis; Therapeutic target.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. - PubMed
-
- Yang X, Yang C, Zhang S, Geng H, Zhu AX, Bernards R, et al. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell. 2024;42(2):180–97. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. - PubMed
-
- Altenberg B, Greulich KO. Genes of Glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014–20. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

