Ophthalmologic Findings in an Induced Model of Holoprosencephaly in Zebrafish
- PMID: 41207878
- PMCID: PMC12597868
- DOI: 10.1002/cne.70113
Ophthalmologic Findings in an Induced Model of Holoprosencephaly in Zebrafish
Abstract
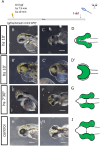
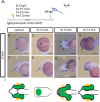
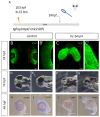
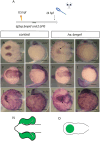
Holoprosencephaly (HPE) is the most frequent developmental disorder of the forebrain. In this condition, the separation of the early precursor domains is hampered. A spectrum of clinical manifestations can be observed, for example, severe forms like alobar HPE and less severe forms like lobar HPE. Ophthalmologic findings that accompany HPE also occur as a spectrum that ranges from ocular hypotelorism and synophthalmia to cyclopia and anophthalmia. In this brief analysis, we made use of a recently established zebrafish model of HPE. This model is based on experimental BMP ligand induction that resulted in anophthalmia. We attenuated the induction protocol to investigate whether the ophthalmologic phenotype can also be attenuated. We found a spectrum of ocular phenotypes: ocular hypotelorism, cases of synophthalmia, and cyclopia.
Keywords: BMP; anophthalmia; bmp4; crypt‐oculoid; cyclopia; holoprosencephaly.
© 2025 The Author(s). The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest
Figures

References
-
- Aono, A. , Hazama M., and Notoya K.. 1995. “Potent Ectopic Bone‐Inducing Activity of Bone Morphogenetic Protein‐4/7 Heterodimer.” Biochemical and Biophysical Research Communications 210: 670–677. - PubMed
-
- Bachiller, D. , Klingensmith J., Kemp C., et al. 2000. “The Organizer Factors Chordin and Noggin Are Required for Mouse Forebrain Development.” Nature 403: 658–661. - PubMed
-
- Bogdanović, O. , Delfino‐Machín M., Nicolás‐Pérez M., et al. 2012. “Numb/Numbl‐Opo Antagonism Controls Retinal Epithelium Morphogenesis by Regulating Integrin Endocytosis.” Developmental Cell 23: 782–795. - PubMed
-
- Bulk, J. , Kyrychenko V., Rensinghoff P. M., Ghaderi Ardekani Z., and Heermann S.. 2023. “Holoprosencephaly With a Special Form of Anophthalmia Result From Experimental Induction of bmp4, Oversaturating BMP Antagonists in Zebrafish.” International Journal of Molecular Sciences 24: 8052. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

