Exposure-Response Analysis of Donidalorsen for the Treatment of Hereditary Angioedema
- PMID: 41208217
- PMCID: PMC12597964
- DOI: 10.1111/cts.70388
Exposure-Response Analysis of Donidalorsen for the Treatment of Hereditary Angioedema
Abstract
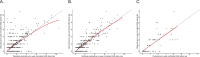
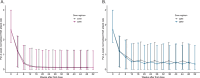
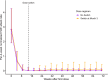
Hereditary angioedema (HAE) is characterized by recurrent attacks of severe tissue swelling. In the OASIS-HAE phase 3 study (NCT05139810), donidalorsen, an RNA-targeted antisense oligonucleotide that reduces prekallikrein production in the liver, significantly reduced HAE attack rates. To characterize the relationship between prekallikrein concentrations and HAE attack rates following donidalorsen and predict the efficacy of potential dosing regimens, an exposure-response model was developed using data from OASIS-HAE. Simulations were conducted to evaluate the following regimens: 80 mg once every 4 weeks (Q4W), 8 weeks (Q8W), 1 month (Q1M), and 2 months (Q2M), and a switch to Q2M dosing for patients who were attack-free for 3 months on the Q1M regimen. The relationship between prekallikrein concentrations and HAE attack rates was well characterized by a sigmoidal Emax (maximum effect) model with baseline attack rate and baseline prekallikrein concentration included as covariates. The average prekallikrein concentration estimated to result in a 90% reduction in attack rate (EC10) was 47.1 mg/L. Predicted percent reductions in attack rates at steady state were similar for Q4W (84.1%) vs. Q1M (82.9%) and Q8W (72.6%) vs. Q2M (70.2%) dosing. Predicted reductions in attack rate remained similar and clinically meaningful in patients who switched from Q1M to Q2M dosing (94.0% in month 1 and 91.3% in month 2 of the 2-month dosing interval at steady state). Overall, exposure-response analyses supported the efficacy of Q1M and Q2M dosing and indicated that switching to Q2M dosing could be a viable approach for patients who are attack-free on the Q1M regimen.
Keywords: exposure–response; modeling; pharmacodynamics; pharmacokinetics; pharmacokinetics–pharmacodynamics.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

