Luminescent Perhalofluoro Trityl Radicals
- PMID: 41208572
- PMCID: PMC12636010
- DOI: 10.1021/jacs.5c16418
Luminescent Perhalofluoro Trityl Radicals
Abstract
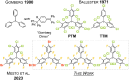
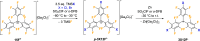
In this proof-of-concept study, we show that polyfluorinated trityl radicals with the, to this date, highest fluorination grade can be accessed in quantitative yields in a straightforward manner starting from the perfluorinated trityl cation. The trityl skeleton is functionalized with trimethylsilyl halides to yield perhalofluoro trityl cations, which are subsequently reduced using commercial zinc powder. In this way, we prepare three perhalofluoro trityl radicals and analyze the impact of the fluorine ligands on their electro-optical properties, revealing some interesting trends. In comparison to literature-known polychlorinated trityl radicals, the new polyfluorinated derivatives exhibit substantially higher fluorescence quantum yields, longer luminescence lifetimes, and an expanded emission range that extends into the yellow spectral region. They further display enhanced photostability under light irradiation. In radical-stained polystyrene nanoparticles, an additional broad emission band in the red-NIR wavelength region is observed, which is attributed to excimer formation. Finally, the stability of the new radicals is investigated under ambient conditions, showing the slow conversion with atmospheric oxygen yielding the respective peroxides, which are characterized by single-crystal X-ray diffraction. All in all, our study extends the present scope of luminescent trityl radicals, as the functionalization of the perfluorinated cationic precursor unlocks the path toward a vast variety of polyfluorinated trityl radicals.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

