Muscle Structure and Function Recovery: Adalimumab-Calcium Channel Synergy in Post-Ischemic Stroke Sarcopenia
- PMID: 41208593
- PMCID: PMC12598305
- DOI: 10.1002/jcsm.70097
Muscle Structure and Function Recovery: Adalimumab-Calcium Channel Synergy in Post-Ischemic Stroke Sarcopenia
Abstract
Background: Adalimumab, a TNF-α inhibitor, is widely used clinically. Recent studies suggest Adalimumab can improve muscle damage after ischemic stroke (IS), but its protective mechanisms remain unclear. This study investigates the effect of adalimumab on muscle structure post-IS and the role of calcium balance in muscle strength, while validating their synergistic effect.
Methods: This study investigates the effects of adalimumab and GV-58 on muscle structure and function in male middle cerebral artery occlusion (MCAO) rat models using behavioural, imaging, pathological and WB experiments. In vitro mechanisms are explored with L6 and primary muscle cells.
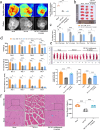
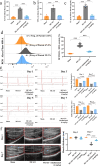
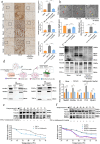
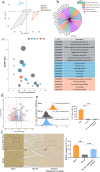
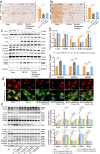
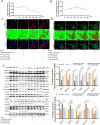
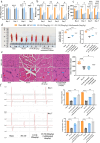
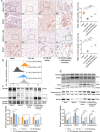
Results: The results of the present study showed a significant decrease in motor function in IS-induced sarcopenia (ISS) rats, as evidenced by shortened length (-47.56%, p < 0.001), reduced weight (-43.79%, p < 0.001) and reduced cross-sectional area of myofibroblasts (-38.58%, p < 0.001) in the soleus muscle as compared to the sham group. Inflammatory factors such as IL-1β, IL-6, TNF-α and reactive oxygen species (ROS) levels were significantly elevated in the muscles of ISS rats (3.10-fold, 3.78-fold, 2.29-fold, 2.80-fold, p < 0.001). Molecular mechanism studies showed that TNF-α, MAFbx and MuRF1 protein expression was down-regulated, and IL-10 and MyoD1 expression was up-regulated in muscle tissues of ISS rats. RNA-seq implicated the Ca2+ signalling pathway in ISS-related muscle weakness. Muscle strength in ISS rats is associated with Ca2+ content and Ca2+ channels, and key excitation-contraction coupling proteins SERCA2, Cav1.1 and RYR1 expression was decreased, whereas Ca2+ sensing proteins STIM1 and CAM expression were compensatory upregulated. Adalimumab treatment significantly reduced muscle inflammation and structural damage in ISS rats, significantly increasing the length (+66.88%, p < 0.001) and weight (+43.92%, p < 0.001) of the soleus muscle and increasing muscle cell cross-sectional area (+53.44%, p < 0.001). Adalimumab also inhibited the expression of MAFbx, MuRF1 and promoted the expression of IL-10 and MyoD1. GV-58 treatment of L6 cells showed that combined administration with adalimumab produced a synergistic effect. Upregulation of key Ca2+ protein expression such as RyR1 and SERCA1 improved the recovery of muscle strength in ISS rats while maintaining muscle structure.
Conclusions: The combination of adalimumab and GV-58 effectively restores muscle function after stroke by inhibiting inflammation and improving calcium channel dysfunction.
Keywords: Ca2+ channels; adalimumab; combination therapy; ischemic stroke; muscle structure.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The authors declare no conflicts of interest.
Figures
References
-
- Li W., Yue T., and Liu Y., “New Understanding of the Pathogenesis and Treatment of Stroke‐Related Sarcopenia,” Biomedicine and Pharmacotherapy 131 (2020): 110721. - PubMed
-
- Qi H., Li X., Zhang X., et al., “Improvement Effect of Chrysanthemum Morifolium Extract on Muscle Atrophy After Ischemic Stroke in Rats,” Chinese Traditional and Herbal Drugs 55 (2024): 6973.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

