Advances in the imaging of pulmonary hypertension
- PMID: 41208912
- PMCID: PMC12594946
- DOI: 10.1016/j.ijcchd.2025.100619
Advances in the imaging of pulmonary hypertension
Abstract
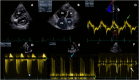
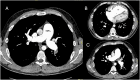
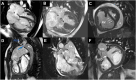
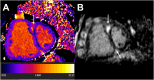
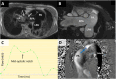
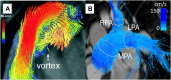
Pulmonary hypertension (PH) is a complex and progressive disorder characterized by elevated pulmonary arterial pressures leading to right ventricular dysfunction and increased morbidity and mortality. Non-invasive imaging, including echocardiography, computed tomography (CT) and cardiovascular magnetic resonance (CMR), plays a crucial role in the diagnosis, risk stratification, and management of PH. The integration of these imaging modalities facilitates a multimodal approach to PH evaluation, enabling more precise diagnosis, improved phenotyping, and better-guided therapeutic decision-making. Echocardiography remains the first-line modality, offering valuable insights into pulmonary artery pressures, right ventricular size and function, and associated cardiac anomalies. Recent developments in speckle-tracking echocardiography and 3D imaging have enhanced its diagnostic and prognostic utility. CT imaging provides detailed evaluation of the pulmonary vasculature, parenchyma, and perfusion, which is essential in distinguishing PH subtypes. CMR is non-invasive, radiation free, and highly sensitive to changes in anatomy and function making it ideal for the long-term follow up of patients with PH. It offers in-depth evaluation of all cardiac chambers as well as pulmonary blood flow assessment and tissue characterisation. In this work we discuss current strengths, limitations, and future directions in these key imaging modalities used for the comprehensive assessment of PH.
© 2025 The Authors.
Conflict of interest statement
The authors report no relationships that could be construed as a conflict of interest other t.
Figures

References
-
- NHS Digital. National audit of pulmonary hypertension 10th annual report, Great Britain, vols. 2018–19., n.d.
-
- Humbert M., Kovacs G., Hoeper M.M., Badagliacca R., Berger R.M.F., Brida M., Carlsen J., Coats A.J.S., Mayer E., Nagavci B., Olsson K.M., Pepke-Zaba J., Quint J.K., Simonneau G., Sitbon O., Toshner M., Delcroix M., Rosenkranz S. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension developed by the task force for the diagnosis and treatment of (ESC) and the european respiratory society (ERS) Eur Heart J. 2022;43:3618–3731. 2022. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

