OCT4-mediated upregulation of DUSP6 promotes metastasis in non-small-cell lung cancer
- PMID: 41210685
- PMCID: PMC12595244
- DOI: 10.7150/jca.108663
OCT4-mediated upregulation of DUSP6 promotes metastasis in non-small-cell lung cancer
Abstract
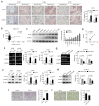
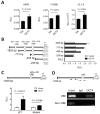
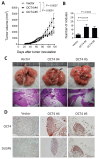
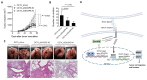
The roles of cancer stem cells and Octamer-binding transcription factor 4 (OCT4) have been implicated in human tumorigenesis and metastasis. However, the role of OCT4 in the metastasis of non-small-cell lung cancer (NSCLC) remains undetermined, especially regarding stem cell-related pathways. Previous research has reported that dual-specificity phosphatase 6 (DUSP6), a mitogen-activated protein kinase (MAPK) phosphatase, is associated with cancer cells that display anti-apoptotic, migratory, and drug-resistance phenotypes. However, the regulation of DUSP6 in NSCLC is unclear. This study focused on the role of OCT4 in NSCLC, particularly its interaction with DUSP6. Here, we show a positive correlation between OCT4 and DUSP6 expression in NSCLC cells. Overexpression of OCT4 increased, whereas knockdown of OCT4 reduced DUSP6 expression. Luciferase reporter and chromatin immunoprecipitation (ChIP) assays revealed that OCT4 transactivated DUSP6 expression by directly binding to the DUSP6 promoter, indicating that DUSP6 is a downstream target of OCT4. Furthermore, knockdown of DUSP6 in OCT4-overexpressing A549 human NSCLC cells decreased cell migration in vitro and reduced tumor growth and pulmonary metastasis in NOD/SCID mice. In conclusion, our findings highlight the importance of the OCT4-DUSP6 pathway in NSCLC progression. Furthermore, the OCT4-DUSP6 axis is a potential therapeutic target for NSCLC.
Keywords: dual-specificity phosphatase 6 (DUSP6); metastasis; non-small-cell lung cancer (NSCLC); octamer-binding transcription factor 4 (OCT4).
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

