Phosphate-Based Approaches for Dechlorination and Treatment of Salt Waste from Electrochemical Processing of Used Nuclear Fuel: A Perspective on Recent Work
- PMID: 41210776
- PMCID: PMC12593076
- DOI: 10.1021/acsomega.5c08801
Phosphate-Based Approaches for Dechlorination and Treatment of Salt Waste from Electrochemical Processing of Used Nuclear Fuel: A Perspective on Recent Work
Abstract
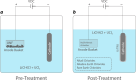
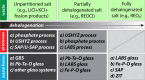
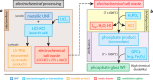
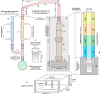
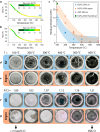
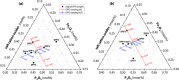
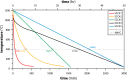
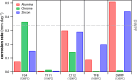
Phosphate-based reagents are being considered by the U.S. Department of Energy (DOE) Office of Nuclear Energy to process halide salt-based nuclear wastes for stabilization prior to disposal. As evidenced by the Experimental Breeder Reactor-II (EBR-II) project, electrochemical processing (pyroprocessing) can be employed to recover uranium and other actinides for reintegration into the nuclear fuel cycle from metallic fuels. The resultant salt-based wastes generated from electrochemical processing of EBR-II fuel contains fission products within a LiCl-KCl eutectic salt that necessitate appropriate disposal. This paper provides an overview of recent efforts to support halide-based salt waste treatment for disposition, as well as a basis for comparison with other related efforts in salt waste treatment through salt partitioning initiatives. The U.S. DOE has selected a phosphate waste form reference material for further investigation and longer-term studies.
© 2025 The Authors. Published by American Chemical Society.
Figures



References
-
- U.S. Congress , Accelerating Deployment of Versatile, Advanced Nuclear For Clean Energy Act. U.S. Government Printing Office: Washington, DC, 2023, Public Law 118-182.
-
- McCloy J. S., Riley B. J., Dixon Wilkins M. C., Evarts J. S., Bussey J., Vienna J. D., Bingham P. A., Gregg D. J., Ojovan M., Schuller S., Uruga K., Perret D., Regnier E., Giboire I., Um W., Xu K., Goel A., Kruger A. A.. International perspectives on glass waste form development for low-level and intermediate-level radioactive waste. Mater. Today. 2024;80:594–618. doi: 10.1016/j.mattod.2024.08.025. - DOI
-
- Ingersoll D. T., Houghton Z. J., Bromm R., Desportes C.. NuScale small modular reactor for Co-generation of electricity and water. Desalin. 2014;340:84–93. doi: 10.1016/j.desal.2014.02.023. - DOI
-
- Schlegel, J. P. ; Bhowmik, P. K. . Chapter 14Small modular reactors. In Nuclear Power Reactor Designs; Wang, J. , Talabi, S. , Leon, S. B. y. , Eds.; Academic Press, 2024; pp 283–308.
-
- International Atomic Energy Agency . Small Modular Reactors: Advances in SMR Developments 2024: Vienna International Centre: Vienna, Austria, 2024.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
