Getting Pabrinex Right: A Multi-cycle Quality Improvement Study Reducing Waste in Thiamine Prescribing at a Scottish District General Hospital
- PMID: 41211257
- PMCID: PMC12594507
- DOI: 10.7759/cureus.96322
Getting Pabrinex Right: A Multi-cycle Quality Improvement Study Reducing Waste in Thiamine Prescribing at a Scottish District General Hospital
Abstract
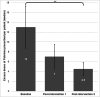
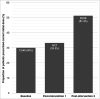
Background Patients with alcohol use disorders (AUD) are at increased risk of Wernicke's encephalopathy (WE) due to thiamine deficiency. At our centre all admitted patients with AUD are assessed for signs, symptoms and risk factors for WE. Such patients are prescribed oral or intravenous (IV) thiamine replacement in accordance with local guidelines; choice of dose and route of administration is determined based on the clinical features present at presentation. Failure to appropriately treat patients with WE may lead to potentially avoidable progression to Korsakoff syndrome. However, unnecessary use of IV formulations, high doses and extended durations of treatment can contribute to unnecessary administrations of IV thiamine, generating financial, environmental and workload costs. In the UK, IV thiamine is usually given in the form of compound vitamin B and C solution (Pabrinex). Aim This quality improvement (QI) project aims to quantify and reduce unnecessary prescription of IV Pabrinex in patients with AUD admitted to a district general hospital using a multi-cycle plan-do-study-act (PDSA) methodology. Materials and methods This retrospective study involved 106 patients admitted over a four-month period. Two education-based prescriber-targeted interventions were performed. Data collection was performed at baseline and after each intervention. Recommended thiamine dose was calculated by reviewing patients' admission documentation for presenting signs, symptoms and demographics. These data were then cross-referenced with local guidelines on the management of patients admitted with AUD to determine the recommended thiamine dosing regimen. The total number of IV Pabrinex doses recommended by guidelines was compared to the actual number prescribed for each patient to determine potentially unnecessary "excess" doses. Primary outcome measures recorded were the number of excess doses prescribed and proportion of patients receiving the correct initial dose. Results Baseline data showed 60% patients had received excess doses of Pabrinex, with a median of 13 excess doses per patient. This represented an excess cost of £35 per patient for medication alone. Following first and second interventions, median excess doses per patient in subsequent cycles fell to seven and then to 4.5, a significant reduction from baseline (p=0.0087). Proportion of patients receiving correct initial prescription showed a non-significant rise from 30% to 51.3% (p=0.12). Conclusions A targeted four-month multi-cycle QI project was carried out to quantify and reduce thiamine overprescription among AUD patients in a Scottish district general hospital. Interventions successfully reduced excess Pabrinex doses given per patient. This study highlights the potential for overprescription in the context of thiamine replacement and findings suggest that low-cost prescriber education strategies can be used to improve practice in this setting. Limitations included the single-centre design and short follow-up period, which limit generalisability. Clinical outcome measures were not recorded here but would be important to consider in future work. Caution should be exercised when aiming to reduce thiamine overprescription to mitigate the risk of underdosing patients who warrant treatment. Following completion of this project, a revised guideline has been implemented for this health board with simplified dosing recommendations, which may yield further improvements in practice.
Keywords: cost reduction; district general hospital; overprescription; pabrinex; prescriber; prescription; quality improvement; thiamine; waste.
Copyright © 2025, Noteman et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Latt N, Dore G. Intern Med J. 2014;44:911–915. - PubMed
-
- Hospital outcomes in medical patients with alcohol-related and non-alcohol-related Wernicke encephalopathy. Rasiah R, Gregoriano C, Mueller B, Kutz A, Schuetz P. Mayo Clin Proc. 2024;99:740–753. - PubMed
-
- Wernicke encephalopathy-clinical pearls. Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Mayo Clin Proc. 2019;94:1065–1072. - PubMed
-
- Diagnosis and treatment of Wernicke's encephalopathy: a systematic literature review. Cantu-Weinstein A, Branning R, Alamir M, Weleff J, Do M, Nero N, Anand A. Gen Hosp Psychiatry. 2024;87:48–59. - PubMed
-
- Glasgow assessment and management of alcohol (GMAWS) adult inpatients GGC. [ Jun; 2025 ]. 2023. https://rightdecisions.scot.nhs.uk/media/2688/id-137-gmaws-adult-inpatie... https://rightdecisions.scot.nhs.uk/media/2688/id-137-gmaws-adult-inpatie...
LinkOut - more resources
Full Text Sources
