Urinary endotrophin as a biomarker for T cell-mediated rejection-associated fibrogenesis in kidney transplant recipients
- PMID: 41215785
- PMCID: PMC12596184
- DOI: 10.1093/ckj/sfaf301
Urinary endotrophin as a biomarker for T cell-mediated rejection-associated fibrogenesis in kidney transplant recipients
Abstract
Background: Endotrophin, a C-terminal pro-collagen type VIα3 fragment, has been shown to correlate with kidney interstitial fibrosis, kidney outcome measures and survival in various kidney diseases and kidney transplantation. In this study we investigated whether endotrophin is associated with fibrogenesis in T cell-mediated rejection (TCMR), allowing its use as a non-invasive biomarker.
Method: Plasma endotrophin and urinary endotrophin (indexed for creatinine) were measured in samples from a cross-sectional study among kidney transplant recipients (KTRs) who underwent indication biopsy after transplantation and enrolled in TransplantLines Biobank and Cohort Study. Endotrophin was measured using the nordicPRO-C6 enzyme-linked immunosorbent assay. Blood and urine were collected on the day of biopsy. In a subset of patients, the biopsy was stained for endotrophin and T cells.
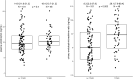
Results: A total of 149 KTRs were included in the analyses. Of them, 48 (32.2%) had TCMR (either borderline, acute, chronic or mixed). Higher urinary endotrophin levels were associated with increased odds of TCMR and the association remained significant after adjustment for other potential confounders, including plasma endotrophin [adjusted odds ratio per doubling 1.38 (95% confidence interval 1.11-1.72), P = .004]. In contrast, higher plasma endotrophin levels were not associated with increased odds of TCMR. T cell density in the biopsy was associated with endotrophin-positive myofibroblasts (ρ = 0.61, P = .045), urinary endotrophin (ρ = 0.67, P = .017) and interstitial fibrosis and tubular atrophy (ρ = 0.61, P = .048).
Conclusion: These data indicate the potential use of urinary endotrophin as a non-invasive biomarker for fibrogenesis in the context of TCMR.
Keywords: biomarkers; collagen α3(VI); endotrophin; graft rejection; kidney transplantation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
D.G.K.R., N.S., F.G. and M.A.K. were full-time employees at Nordic Bioscience and D.G.K.R., F.G. and M.A.K. were stockholders at Nordic Bioscience at the time the research was performed. Nordic Bioscience is a privately owned, small- to medium-size enterprise partly focused on the development of biomarkers and owns the patent for the ELISA used to measure endotrophin levels. The funders had no role in data collection, analysis or interpretation; trial design; patient recruitment; or any aspect pertinent to the study or the decision to submit it for publication. There was no payment for writing this article by a pharmaceutical company or other agencies. No authors received fees, bonuses or other benefits for the work described in this article and Nordic Bioscience did not have any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. All other authors of this manuscript have no conflicts of interest to disclose.
Figures



References
LinkOut - more resources
Full Text Sources

