Cell encapsulated biomaterials for translational medicine
- PMID: 41216376
- PMCID: PMC12596701
- DOI: 10.1016/j.bioactmat.2025.10.021
Cell encapsulated biomaterials for translational medicine
Abstract
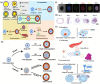
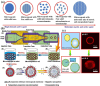
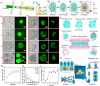
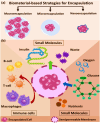
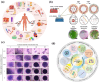
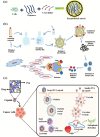
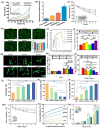
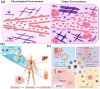
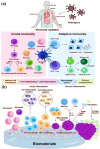
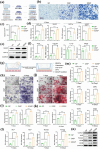
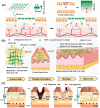
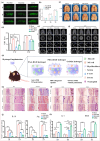
Biomaterial supported cell encapsulation matrices have demonstrated superior properties for enhancing biological functionality, making them highly significant for translational medicine across multiple therapeutic applications. This review examined how biomaterials interact with cellular therapies, including stem cells, immune cells, and fibroblasts across single-cell, multicellular, and core-shell structures. The biomaterial capsule plays a key role in improving cell viability, immune protection, and supporting tissue-specific interactions. Furthermore, this review highlights current trends in microfluidics, 3D printing, in situ preparation, and electrospraying self-assembly, each method offering different advantages for cell encapsulation matrices. Microfluidics allows precise control of capsule size and uniformity, making it suitable for single-cell and core-shell encapsulation. The 3D printing technologies empower accurate cell placement to build multicellular structures that mimic native tissue organization. In situ preparation directly encapsulates cells within the target tissue. Collectively, these techniques significantly influence the physical, chemical, and biological properties of encapsulated cells. Additionally, we discuss various biomaterials including natural proteins, polysaccharides, and synthetic polymers, each material offers unique benefits in terms of biocompatibility and biodegradability. The integration of living cells with biomaterial matrix cell encapsulation systems greatly exhibits mechanical strength, high porosity, and controlled drug release. Importantly, this review emphasises the dual role of the biomaterial capsule in cancer therapy, which enhances anti-tumor immune responses and promotes tissue regeneration, with a focus on bone, skin, neural tissue, liver, vascular structures, and skeletal muscle repair. In conclusion, cell-encapsulated biomaterials are a versatile platform supporting both cancer immunotherapy and regenerative medicine, underscoring their wide range of biomedical applications.
Keywords: Biomaterials; Cancer therapy; Cell encapsulation; Immune cell; Tissue regeneration.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures













References
-
- Gonzalez-Pujana A., Santos E., Orive G., Pedraz J.L., Hernandez R.M. Cell microencapsulation technology: current vision of its therapeutic potential through the administration routes. J. Drug Deliv. Sci. Technol. 2017;42:49–62. doi: 10.1016/j.jddst.2017.03.028. - DOI
-
- Chen Q.W., Zhang X.-Z. Living biomaterials: fabrication strategies and biomedical applications. Acc. Mater. Res. 2024;5:1440–1452. doi: 10.1021/accountsmr.4c00258. - DOI
-
- Shao D., Gao Q., Sheng Y., Li S., Kong Y. Construction of a dual-responsive dual-drug delivery platform based on the hybrids of mesoporous silica, sodium hyaluronate, chitosan and oxidized sodium carboxymethyl cellulose. Int. J. Biol. Macromol. 2022;202:37–45. doi: 10.1016/j.ijbiomac.2022.01.033. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

