Protocol for a multicenter, cluster-randomized, stepped-wedge, implementation trial of a prehospital sepsis protocol
- PMID: 41220003
- PMCID: PMC12607186
- DOI: 10.1186/s13063-025-09172-3
Protocol for a multicenter, cluster-randomized, stepped-wedge, implementation trial of a prehospital sepsis protocol
Abstract
Background: Sepsis is a common, life-threatening medical emergency in which early recognition and treatment are cornerstones of management. Best practice guidelines recommend standardized sepsis screening in hospitals to facilitate early recognition. In contrast, very little is known about the efficacy and safety of standardized sepsis screening in the prehospital Emergency Medical Services (EMS) environment. To help address this knowledge gap, this study protocol will evaluate the impact of implementing an evidence-based sepsis protocol in ambulances.
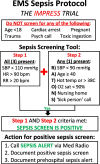
Methods: We plan to conduct a multicenter, stepped-wedge, cluster-randomized trial at 3 sites and enroll 984 participants over 24 months. EMS personnel will be trained to screen all eligible EMS patients using a sepsis screening and early notification protocol. Study inclusion criteria include all the following: (1) EMS systolic blood pressure < 110 mmHg, (2) EMS pulse > 90 beats/min, (3) EMS respiratory rate > 20 breaths/min, and (4) a positive sepsis screen based on the EMS sepsis protocol. To evaluate the clinical impact of the EMS sepsis protocol on patient care, the primary outcome is time to first antibiotic administration in the ED (emergency department) among patients with sepsis (a key treatment intervention in patients with sepsis). To evaluate the potential risk of the EMS sepsis protocol, the surrogate primary safety outcome is the total number of antibiotic DOT (days of therapy) during the first 7 days of hospitalization among patients without sepsis (i.e., false positive sepsis screens).
Discussion: Successful completion of this trial will expand our understanding of prehospital sepsis screening as a standardized approach to prehospital sepsis care.
Trial registration: ClinicalTrials.gov Identifier: NCT05502107. Date of first posting: 08-16-2022. https://clinicaltrials.gov/ct2/show/NCT05502107 .
Keywords: Prehospital; Protocol; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate {24}: This study has been deemed a minimal risk study and granted waiver of informed consent by the central IRB of record, WCG. Consent for publication {32}: Not applicable. Competing interests {28}: JES’s institution receives grant funding from the Department of Defense, the CDC Foundation, the CDC, and Regeneron. JES’s institution also receives funding from the society of critical care medicine for work as an editor.
References
-
- Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49(11):e1063–143. 10.1097/CCM.0000000000005337. - PubMed
-
- Groenewoudt M, Roest AA, Leijten FM, Stassen PM. Septic patients arriving with emergency medical services: a seriously ill population. Eur J Emerg Med. 2014;21(5):330–5. 10.1097/MEJ.0000000000000091. - PubMed
-
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96. 10.1097/01.CCM.0000217961.75225.E9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous