Effectiveness of nirsevimab against hospitalisation for RSV-bronchiolitis during high RSV-B circulation in the second year of nationwide implementation in France: a test-negative case-control study
- PMID: 41220615
- PMCID: PMC12598395
- DOI: 10.1016/j.lanepe.2025.101443
Effectiveness of nirsevimab against hospitalisation for RSV-bronchiolitis during high RSV-B circulation in the second year of nationwide implementation in France: a test-negative case-control study
Abstract
Background: Nirsevimab was first introduced in September 2023, showing strong real-world effectiveness in preventing hospitalised RSV-bronchiolitis. RSV-A circulation predominated during 2023-2024, whereas RSV-B was frequent in 2024-2025. Recent reports indicated resistance to nirsevimab in RSV-B strains in France. We aimed to assess the effectiveness of large-scale nirsevimab implementation against hospitalised RSV-bronchiolitis during high RSV-B circulation.
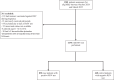
Methods: A test-negative case-control study was conducted using a national hospital-based surveillance system. All children <12 months hospitalised for bronchiolitis and tested for RSV in 12 French hospitals from October 10, 2024 to March 15, 2025, were included. Cases were RSV-positive bronchiolitis; controls were RSV-negative bronchiolitis. Effectiveness was assessed by a multivariable logistic regression model adjusted for confounders (sex, gestational age at birth, birth weight, risk factors for severe bronchiolitis, month of diagnosis, and medical centre). A range of sensitivity analyses was conducted.
Findings: 1270 patients were included; among which 830 (65.3%) were RSV-positive and 440 (34.7%) were RSV-negative bronchiolitis. The male-to-female sex ratio was 1.35, and the median age was 3 months (IQR: 1.5-5.6). Among cases, 182 (22.0%) received nirsevimab, compared to 282 (64.1%) for controls. Adjusted effectiveness against RSV-bronchiolitis was 84.9% (95% CI, 80.0-88.6). Subgroup analyses by age and severity (intensive care unit admission, respiratory support) showed consistent results, along with sensitivity analyses.
Interpretation: Despite high RSV-B circulation, which has recently been identified to carry mutations potentially inducing resistance to nirsevimab, the effectiveness of the second national nirsevimab campaign against hospitalisation for RSV-bronchiolitis remained high.
Funding: The study received financial support from Sanofi, AstraZeneca, and the 2023 ATIP-Avenir grant from the National Institute of Health and Medical Research (Inserm).
Keywords: Effectiveness; Nirsevimab; Paediatrics; RSV-bronchiolitis; Test-negative study.
© 2025 The Author(s).
Conflict of interest statement
RB declares receiving fees from Sanofi and MSD for medical conferences or scientific meetings. CL declares receiving travel grants from MSD, Pfizer and fees from Sanofi, GSK, MSD, and Pfizer for scientific meetings and expert board participation. RC reports personal fees and non-financial support from Pfizer and personal fees from Cerballiance, GSK, Merck, Pfizer, Sanofi, Viatris outside the submitted work. NO declares receiving travel grants from MSD, Pfizer, Sanofi, and GSK. VG declares receiving fees from Sanofi and MSD for medical conferences and expert group. EL declares receiving travel grants from GSK and fees for medical conference from MSD. EJ declares receiving travel grants from Sanofi, GRIFOLS, MSD and fees from Sanofi, LFB, GSK, Pfizer for scientific meetings and expert board participation. IH declares receiving grants drop Pfizer, Sanofi, and MSD, and consulting fees from Cerballiance and payment for symposiums from GSK. FD déclares receiving travel Grant from MSD and being board member for Sanofi and MSD. All other authors have no conflicts of interest to disclose.
Figures

References
-
- Villavicencio F., Perin J., Eilerts-Spinelli H., et al. Global, regional, and national causes of death in children and adolescents younger than 20 years: an open data portal with estimates for 2000–21. Lancet Glob Health. 2024;12(1):e16–e17. - PubMed
-
- Hammitt L.L., Dagan R., Yuan Y., et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837–846. - PubMed
-
- Haute Autorité de Santé Décision n°2023.0315/DC/SBP du 14 septembre 2023 du collège de la Haute Autorité de santé portant adoption du document intitulé « Réponse rapide: Nirsévimab (BEYFORTUS®) dans la prévention des bronchiolites à virus respiratoire syncytial (VRS) chez les nouveau-nés et les nourrissons » [Internet] https://www.has-sante.fr/jcms/p_3461229/fr/decision-n2023-0315/dc/sbp-du... Available from:
LinkOut - more resources
Full Text Sources

