Etoricoxib and its hidden risks: a case-based review of dermatological, hematological, and cardiovascular complications
- PMID: 41220918
- PMCID: PMC12598108
- DOI: 10.17179/excli2025-8751
Etoricoxib and its hidden risks: a case-based review of dermatological, hematological, and cardiovascular complications
Abstract
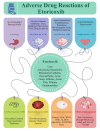
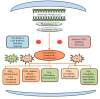
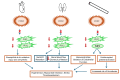
This review analyzes case reports of adverse drug reactions (ADRs) attributed to etoricoxib, with particular emphasis on Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), fixed drug eruptions (FDE), atrial fibrillation, hypertension, thrombocytopenia (TP), immune hemolytic anemia (IHA), acute generalized exanthematous pustulosis (AGEP), maculopapular rash, pretibial erythema with edema, and reversible cerebral vasoconstriction syndrome (RCVS). Although infrequent, these severe hypersensitivity and cardiovascular events pose significant clinical risks due to their association with substantial morbidity and, in some cases, mortality. The primary aim of this review is to consolidate available clinical evidence to evaluate the causality, characteristic clinical presentations, and broader safety implications of etoricoxib in relation to these adverse outcomes. While SJS/TEN are marked by widespread epidermal necrosis and detachment, FDE typically recurs at fixed sites with residual pigmentation. Hematological complications such as drug-induced (TP) and Drug-induced IHA have also been reported, presenting as sudden platelet decline or severe hemolysis, respectively. These adverse effects often appear within hours to weeks of initiating therapy. Cutaneous manifestations, including exanthematous pustulosis and maculopapular rashes, further complicate the drug's safety profile. Etoricoxib's pro-thrombotic potential, possibly linked to COX-2 selectivity, remains a cardiovascular concern. Causality assessments via the Naranjo Scale and WHO-UMC often support a probable link. These findings underscore the necessity for careful evaluation of patient history, immediate drug discontinuation upon clinical suspicion, and strengthened pharmacovigilance systems to better capture and characterize the full range of these rare yet serious reactions. See also the graphical abstract(Fig. 1).
Keywords: Stevens-Johnson syndrome; atrial fibrillation; etoricoxib; fixed drug eruptions; hypertension; toxic epidermal necrolysis.
Copyright © 2025 Ansari et al.
Figures









References
-
- Ahamed H, Mohan MM, Shobha P, Ramani PT, Harsha Devi S. A Case Report of Stevens-Johnson Syndrome Probably Due to Etoricoxib. Int J Med Sci Curr Res (IJMSCR) 2024;7:380–382.
-
- Ahmadi M, Bekeschus S, Weltmann KD, von Woedtke T, Wende K. Non-steroidal anti-inflammatory drugs: recent advances in the use of synthetic COX-2 inhibitors. RSC Med Chem. 2022;13:471–96. doi: 10.1039/D1MD00280E. Available from: http://dx.doi.org/10.1039/D1MD00280E. - DOI - PMC - PubMed
-
- Ali KA, Maity A, Roy SD, Das Pramanik S, Pratim Das P, Shaharyar MA. Insight into the mechanism of steroidal and non-steroidal anti-inflammatory drugs. In: Kazmi I, Karmakar S, Shaharyar Md. Adil, Afzal M, Al-Abbasi FA, editors. ow Synthetic Drugs Work: Insights into Molecular Pharmacology of Classic and New Pharmaceuticals. Amsterdam: Academic Press; 2023. pp. 61–94. Available from: http://dx.doi.org/10.1016/B978-0-323-99855-0.00004-X. - DOI
-
- Amstutz U, Shear NH, Rieder MJ, Hwang S, Fung V, Nakamura H, et al. Recommendations for HLA-B15:02 and HLA-A31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55:496–506. doi: 10.1111/EPI.12564. Available from: http://dx.doi.org/10.1111/EPI.12564. - DOI - PubMed
-
- Anderson HJ, Lee JBA, Anderson HJ, Lee JBA. A Review of Fixed Drug Eruption with a Special Focus on Generalized Bullous Fixed Drug Eruption. Medicina. 2021;57:925. doi: 10.3390/MEDICINA57090925. Available from: http://dx.doi.org/10.3390/MEDICINA57090925. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
