Anti-diuretic hormone ITP signals via a guanylate cyclase receptor to modulate systemic homeostasis in Drosophila
- PMID: 41222455
- PMCID: PMC12611267
- DOI: 10.7554/eLife.97043
Anti-diuretic hormone ITP signals via a guanylate cyclase receptor to modulate systemic homeostasis in Drosophila
Abstract
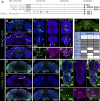
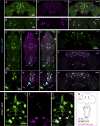
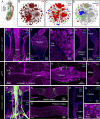
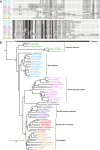
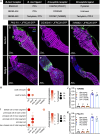
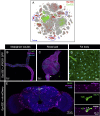
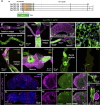
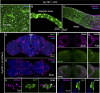
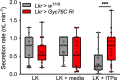
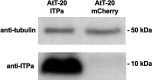
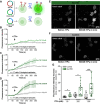
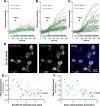
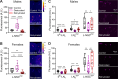
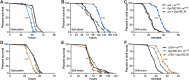
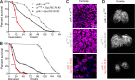
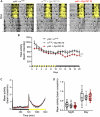
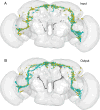
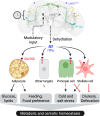
Insects have evolved a variety of neurohormones that enable them to maintain nutrient and osmotic homeostasis. Here, we characterized the ion transport peptide (ITP) signaling system in Drosophila. The Drosophila ITP gene can generate three different peptide isoforms: ITP amidated (ITPa) and two ITP-like (ITPL1 and ITPL2) isoforms. We comprehensively characterized the expression of all three ITP isoforms in the nervous system and peripheral tissues. Our analyses reveal widespread expression of ITP isoforms. Moreover, we show that ITPa-producing neurons are activated and release ITPa during dehydration. Furthermore, recombinant Drosophila ITPa inhibits diuretic peptide-induced renal tubule secretion ex vivo, thus confirming its role as an anti-diuretic hormone. Using a phylogenetic-driven approach, an ex vivo secretion assay and a heterologous mammalian cell-based assay, we identified and functionally characterized Gyc76C, a membrane guanylate cyclase, as a bona fide Drosophila ITPa receptor. Extensive anatomical mapping of Gyc76C reveals that it is highly expressed in larval and adult tissues associated with osmoregulation (renal tubules and rectum) and metabolic homeostasis (fat body). Consistent with this expression, knockdown of Gyc76C in renal tubules impacts tolerance to osmotic and ionic stresses, whereas knockdown specifically in the fat body impacts feeding, nutrient homeostasis, and associated behaviors. We also complement receptor knockdown experiments with ITP knockdown and ITPa overexpression in ITPa-producing neurons. Lastly, we utilized connectomics and single-cell transcriptomics to identify pathways via which ITP neurons integrate hygrosensory inputs and interact with other homeostatic hormonal pathways. Taken together, our systematic characterization of ITP signaling establishes a tractable system to decipher how a small set of neurons integrates diverse inputs to orchestrate systemic homeostasis in Drosophila.
Keywords: D. melanogaster; atrial natriuretic peptide; computational biology; connectomics; endocrine signaling; metabolism; neuropeptide; neuroscience; osmoregulation; systems biology.
© 2024, Gera et al.
Conflict of interest statement
JG, MA, HN, AB, FS, LT, TM, SK, DN, MO, JP No competing interests declared, MZ Reviewing editor, eLife
Figures










Update of
- doi: 10.1101/2024.02.07.579245
- doi: 10.7554/eLife.97043.1
- doi: 10.7554/eLife.97043.2
References
MeSH terms
Substances
Grants and funding
- Discovery Grant/Natural Sciences and Engineering Research Council of Canada
- 20H03246/Japan Society for the Promotion of Science
- ZA1296/1-1/Deutsche Forschungsgemeinschaft
- U24 NS126935/NS/NINDS NIH HHS/United States
- P20 GM103440/GM/NIGMS NIH HHS/United States
- GM103440/GM/NIGMS NIH HHS/United States
- RF1 MH117815/MH/NIMH NIH HHS/United States
- P20GM130459/GM/NIGMS NIH HHS/United States
- 2015-04626/Vetenskapsrådet
- P20 GM130459/GM/NIGMS NIH HHS/United States
- PGS-D/Natural Sciences and Engineering Research Council of Canada
- CGS-D/Natural Sciences and Engineering Research Council of Canada
- Early Researcher Award/Ontario Ministry of Research and Innovation
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

